当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen generation from methanol at near-room temperature
Chemical Science ( IF 7.6 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1039/c7sc01778b Yangbin Shen 1, 2 , Yulu Zhan 1 , Shuping Li 1 , Fandi Ning 1 , Ying Du 1 , Yunjie Huang 3 , Ting He 1 , Xiaochun Zhou 1, 4
Chemical Science ( IF 7.6 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1039/c7sc01778b Yangbin Shen 1, 2 , Yulu Zhan 1 , Shuping Li 1 , Fandi Ning 1 , Ying Du 1 , Yunjie Huang 3 , Ting He 1 , Xiaochun Zhou 1, 4
Affiliation
|
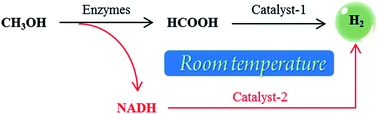
As a promising hydrogen storage medium methanol has many advantages such as a high hydrogen content (12.5 wt%) and low-cost. However, conventional methanol–water reforming methods usually require a high temperature (>200 °C). In this research, we successfully designed an effective strategy to fully convert methanol to hydrogen for at least 1900 min (∼32 h) at near-room temperature. The strategy involves two main procedures, which are CH3OH → HCOOH → H2 and CH3OH → NADH → H2. HCOOH and the reduced form of nicotinamide adenine dinucleotide (NADH) are simultaneously produced through the dehydrogenation of methanol by the cooperation of alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH). Subsequently, HCOOH is converted to H2 by a new iridium polymer complex catalyst and an enzyme mimic is used to convert NADH to H2 and nicotinamide adenine dinucleotide (NAD+). NAD+ can then be reconverted to NADH by repeating the dehydrogenation of methanol. This strategy and the catalysts invented in this research can also be applied to hydrogen production from other small organic molecules (e.g. ethanol) or biomass (e.g. glucose), and thus will have a high impact on hydrogen storage and applications.
中文翻译:

近室温下甲醇制氢
甲醇作为一种有前景的储氢介质具有氢含量高(12.5 wt%)和低成本等许多优点。然而,传统的甲醇-水重整方法通常需要高温(>200℃)。在这项研究中,我们成功设计了一种有效的策略,可以在接近室温的情况下将甲醇完全转化为氢气至少 1900 分钟(~32 小时)。该策略涉及两个主要过程,即CH 3 OH→HCOOH→H 2和CH 3 OH→NADH→H 2 。在乙醇脱氢酶(ADH)和乙醛脱氢酶(ALDH)的配合下,甲醇脱氢,同时产生HCOOH和还原型烟酰胺腺嘌呤二核苷酸(NADH)。随后,HCOOH 通过新型铱聚合物络合物催化剂转化为 H 2 ,并使用酶模拟物将 NADH 转化为 H 2和烟酰胺腺嘌呤二核苷酸 (NAD + )。然后,通过重复甲醇脱氢,NAD +可以重新转化为 NADH。该策略和本研究发明的催化剂也可以应用于从其他小有机分子(例如乙醇)或生物质(例如葡萄糖)生产氢气,因此将对氢的储存和应用产生重大影响。
更新日期:2017-09-20
中文翻译:

近室温下甲醇制氢
甲醇作为一种有前景的储氢介质具有氢含量高(12.5 wt%)和低成本等许多优点。然而,传统的甲醇-水重整方法通常需要高温(>200℃)。在这项研究中,我们成功设计了一种有效的策略,可以在接近室温的情况下将甲醇完全转化为氢气至少 1900 分钟(~32 小时)。该策略涉及两个主要过程,即CH 3 OH→HCOOH→H 2和CH 3 OH→NADH→H 2 。在乙醇脱氢酶(ADH)和乙醛脱氢酶(ALDH)的配合下,甲醇脱氢,同时产生HCOOH和还原型烟酰胺腺嘌呤二核苷酸(NADH)。随后,HCOOH 通过新型铱聚合物络合物催化剂转化为 H 2 ,并使用酶模拟物将 NADH 转化为 H 2和烟酰胺腺嘌呤二核苷酸 (NAD + )。然后,通过重复甲醇脱氢,NAD +可以重新转化为 NADH。该策略和本研究发明的催化剂也可以应用于从其他小有机分子(例如乙醇)或生物质(例如葡萄糖)生产氢气,因此将对氢的储存和应用产生重大影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号