European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-09-20 , DOI: 10.1016/j.ejmech.2017.09.031 Danylo Kaminskyy 1 , Anna Kryshchyshyn 1 , Roman Lesyk 1
|
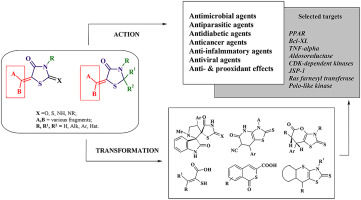
The presented review is an attempt to summarize a huge volume of data on 5-ene-4-thiazolidinones being a widely studied class of small molecules used in modern organic and medicinal chemistry. The manuscript covers approaches to the synthesis of 5-ene-4-thiazolidinone derivatives: modification of the C5 position of the basic core; synthesis of the target compounds in the one-pot or multistage reactions or transformation of other related heterocycles. The most prominent pharmacological profiles of 5-ene derivatives of different 4-thiazolidinone subtypes belonging to hit-, lead-compounds, drug-candidates and drugs as well as the most studied targets have been discussed. Currently target compounds (especially 5-en-rhodanines) are assigned as frequent hitters or pan-assay interference compounds (PAINS) within high-throughput screening campaigns. Nevertheless, the crucial impact of the presence/nature of C5 substituent (namely 5-ene) on the pharmacological effects of 5-ene-4-thiazolidinones was confirmed by the numerous listed findings from the original articles. The main directions for active 5-ene-4-thiazolidinones optimization have been shown: i) complication of the fragment in the C5 position; ii) introduction of the substituents in the N3 position (especially fragments with carboxylic group or its derivatives); iii) annealing in complex heterocyclic systems; iv) combination with other pharmacologically attractive fragments within hybrid pharmacophore approach. Moreover, the utilization of 5-ene-4-thiazolidinones in the synthesis of complex compounds with potent pharmacological application is described. The chemical transformations cover mainly the reactions which involve the exocyclic double bond in C5 position of the main core and correspond to the abovementioned direction of the 5-ene-4-thiazolidinone modification.
中文翻译:

5-Ene-4-噻唑烷酮 – 药物化学的有效工具
本综述试图总结有关 5-ene-4-thiazolidinones 的大量数据,5-ene-4-thiazolidinones 是现代有机化学和药物化学中广泛研究的一类小分子。该手稿涵盖了 5-ene-4-thiazolidinone 衍生物的合成方法:基本核心 C5 位置的修饰;通过一锅或多级反应合成目标化合物或其他相关杂环的转化。讨论了属于命中化合物、先导化合物、候选药物和药物的不同 4-噻唑烷酮亚型的 5-烯衍生物的最突出的药理学特征以及研究最多的靶标。目前,目标化合物(尤其是 5-en-绕丹宁)在高通量筛选活动中被指定为频繁击中者或泛测定干扰化合物 (PAINS)。尽管如此,C5取代基(即5-烯)的存在/性质对5-烯-4-噻唑烷酮的药理作用的关键影响已被原始文章中列出的众多发现所证实。活性 5-ene-4-thiazolidinones 优化的主要方向已显示: i ) C5 位片段的复杂化; ii )在N3位引入取代基(特别是带有羧基或其衍生物的片段); iii ) 在复杂杂环系统中退火; iv ) 在混合药效基团方法中与其他药理学上有吸引力的片段组合。此外,还描述了 5-ene-4-thiazolidinones 在合成具有有效药理学应用的复杂化合物中的用途。 化学转化主要包括涉及主核C5位环外双键的反应,对应于上述5-烯-4-噻唑烷酮修饰的方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号