当前位置:
X-MOL 学术
›
Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of adding LiBF4 on the glass-transition kinetics of 1,2-propanediol
Chemical Physics ( IF 2.3 ) Pub Date : 2017-09-20 , DOI: 10.1016/j.chemphys.2017.07.013 Yukio Terashima , Kiyoshi Takeda
Chemical Physics ( IF 2.3 ) Pub Date : 2017-09-20 , DOI: 10.1016/j.chemphys.2017.07.013 Yukio Terashima , Kiyoshi Takeda
|
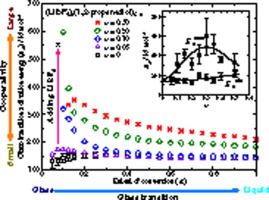
By applying an isoconversional method to differential-scanning calorimetry (DSC) data, we have discovered that the addition of LiBF4 significantly affects the activation energy Eα of the glass transition of 1,2-propanediol. Depending upon its concentration, the dynamics of the glass transition are affected more by adding LiBF4 at an early stage of the glass-to-liquid transition rather than at later stages. As the mole fraction x of LiBF4 increases, the value of Eα initially increases, but it decreases dramatically during the glass transition. The abrupt decline in Eα suggests that the addition of LiBF4 breaks cooperative rearranging motions into smaller parts. The expansion of cooperativity, and its fragmentation with increasing temperature, can be explained in terms of competition between the hydrogen-bond networks of the alcohol solvent and the ionic interactions due to the added salt. The variability of Eα with temperature is found to correlate exponentially with the dynamic fragility.
中文翻译:

LiBF 4的添加对1,2-丙二醇的玻璃化动力学的影响
通过施加等转化率的方法来差分扫描量热法(DSC)的数据,我们已经发现,在加入的LiBF的4显著影响活化能Ë α 1,2-丙二醇的玻璃化转变的。取决于其浓度,在玻璃-液体转变的早期而不是在后期,加入LiBF 4会更多地影响玻璃转变的动力学。作为摩尔分数X的的LiBF 4的增加,的值ê α最初增加,但是它的玻璃化转变期间显着降低。在急速下滑Ë α表明,添加的LiBF的4将协作重排动作分解为较小的部分。协同作用的扩展及其随温度升高而断裂的现象,可以用醇溶剂的氢键网络之间的竞争和由于添加盐引起的离子相互作用来解释。的可变性ë α与温度被发现与动态脆性指数地相关。
更新日期:2017-09-20
中文翻译:

LiBF 4的添加对1,2-丙二醇的玻璃化动力学的影响
通过施加等转化率的方法来差分扫描量热法(DSC)的数据,我们已经发现,在加入的LiBF的4显著影响活化能Ë α 1,2-丙二醇的玻璃化转变的。取决于其浓度,在玻璃-液体转变的早期而不是在后期,加入LiBF 4会更多地影响玻璃转变的动力学。作为摩尔分数X的的LiBF 4的增加,的值ê α最初增加,但是它的玻璃化转变期间显着降低。在急速下滑Ë α表明,添加的LiBF的4将协作重排动作分解为较小的部分。协同作用的扩展及其随温度升高而断裂的现象,可以用醇溶剂的氢键网络之间的竞争和由于添加盐引起的离子相互作用来解释。的可变性ë α与温度被发现与动态脆性指数地相关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号