当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites
Water Research ( IF 11.4 ) Pub Date : 2017-09-20 , DOI: 10.1016/j.watres.2017.09.034 Baile Wu , Liping Fang , John D. Fortner , Xiaohong Guan , Irene M.C. Lo
Water Research ( IF 11.4 ) Pub Date : 2017-09-20 , DOI: 10.1016/j.watres.2017.09.034 Baile Wu , Liping Fang , John D. Fortner , Xiaohong Guan , Irene M.C. Lo
|
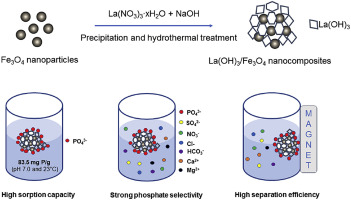
The use of lanthanum (La)-based materials for phosphate removal from water and wastewater has received increasing attention. However, challenges remain to enhance phosphate sorption capacities and recover La-based sorbents. In this study, magnetic La(OH)3/Fe3O4 nanocomposites with varied La-to-Fe mass ratios were synthesized through a precipitation and hydrothermal method. Based upon preliminary screening of synthesized La(OH)3/Fe3O4 nanocomposites in terms of phosphate sorption capacity and La content, La(OH)3/Fe3O4 nanocomposite with a La-to-Fe mass ratio of 4:1 was chosen for further characterization and evaluation. Specifically, for these materials, magnetic separation efficiency, phosphate sorption kinetics and isotherm behavior, and solution matrix effects (e.g., coexisting ions, solution pH, and ionic strength) are reported. The developed La(OH)3/Fe3O4 (4:1) nanocomposite has an excellent magnetic separation efficiency of >98%, fast sorption kinetics of 30 min, high sorption capacity of 83.5 mg P/g, and strong selectivity for phosphate in presence of competing ions. Phosphate uptake by La(OH)3/Fe3O4 (4:1) was pH-dependent with the highest sorption capacities observed over a pH range of 4–6. The ionic strength of the solution had little interference with phosphate sorption. Sorption-desorption cyclic experiments demonstrated the good reusability of the La(OH)3/Fe3O4 (4:1) nanocomposite. In a real treated wastewater effluent with phosphate concentration of 1.1 mg P/L, 0.1 g/L of La(OH)3/Fe3O4 (4:1) efficiently reduced the phosphate concentration to below 0.05 mg P/L. Electrostatic attraction and inner-sphere complexation between La(OH)3 and P via ligand exchange were identified as the sorption mechanisms of phosphate by La(OH)3/Fe3O4 (4:1).
中文翻译:

磁性可回收La(OH)3 / Fe 3 O 4纳米复合材料从废水中高效选择性去除磷酸盐
镧(La)基材料用于从水和废水中去除磷酸盐的用途受到了越来越多的关注。然而,提高磷酸盐吸附能力和回收基于La的吸附剂仍然是挑战。在这项研究中,通过沉淀和水热法合成了具有不同的La-Fe质量比的磁性La(OH)3 / Fe 3 O 4纳米复合材料。基于对磷酸盐吸附能力和La含量的合成La(OH)3 / Fe 3 O 4纳米复合材料的初步筛选,La(OH)3 / Fe 3 O 4选择La与Fe的质量比为4:1的纳米复合材料,以进行进一步的表征和评估。具体而言,对于这些材料,报道了磁分离效率,磷酸盐吸附动力学和等温线行为以及溶液基质效应(例如,共存离子,溶液pH和离子强度)。研发的La(OH)3 / Fe 3 O 4(4:1)纳米复合材料具有> 98%的出色磁分离效率,30分钟的快速吸附动力学,83.5 mg P / g的高吸附容量以及对H2O的强选择性。竞争离子存在下的磷酸盐。La(OH)3 / Fe 3 O 4吸收磷酸盐(4:1)是pH依赖性的,在4–6的pH范围内观察到最高的吸附能力。溶液的离子强度几乎不影响磷酸盐的吸附。吸附-解吸循环实验表明,La(OH)3 / Fe 3 O 4(4:1)纳米复合材料具有良好的可重复使用性。在磷酸盐浓度为1.1 mg P / L的实际处理废水中,0.1 g / L La(OH)3 / Fe 3 O 4(4:1)有效地将磷酸盐浓度降低至0.05 mg P / L以下。La(OH)3和P之间通过配体交换产生的静电吸引和内球络合被确定为La(OH)3对磷酸盐的吸附机理/ Fe 3 O 4(4:1)。
更新日期:2017-09-20
中文翻译:

磁性可回收La(OH)3 / Fe 3 O 4纳米复合材料从废水中高效选择性去除磷酸盐
镧(La)基材料用于从水和废水中去除磷酸盐的用途受到了越来越多的关注。然而,提高磷酸盐吸附能力和回收基于La的吸附剂仍然是挑战。在这项研究中,通过沉淀和水热法合成了具有不同的La-Fe质量比的磁性La(OH)3 / Fe 3 O 4纳米复合材料。基于对磷酸盐吸附能力和La含量的合成La(OH)3 / Fe 3 O 4纳米复合材料的初步筛选,La(OH)3 / Fe 3 O 4选择La与Fe的质量比为4:1的纳米复合材料,以进行进一步的表征和评估。具体而言,对于这些材料,报道了磁分离效率,磷酸盐吸附动力学和等温线行为以及溶液基质效应(例如,共存离子,溶液pH和离子强度)。研发的La(OH)3 / Fe 3 O 4(4:1)纳米复合材料具有> 98%的出色磁分离效率,30分钟的快速吸附动力学,83.5 mg P / g的高吸附容量以及对H2O的强选择性。竞争离子存在下的磷酸盐。La(OH)3 / Fe 3 O 4吸收磷酸盐(4:1)是pH依赖性的,在4–6的pH范围内观察到最高的吸附能力。溶液的离子强度几乎不影响磷酸盐的吸附。吸附-解吸循环实验表明,La(OH)3 / Fe 3 O 4(4:1)纳米复合材料具有良好的可重复使用性。在磷酸盐浓度为1.1 mg P / L的实际处理废水中,0.1 g / L La(OH)3 / Fe 3 O 4(4:1)有效地将磷酸盐浓度降低至0.05 mg P / L以下。La(OH)3和P之间通过配体交换产生的静电吸引和内球络合被确定为La(OH)3对磷酸盐的吸附机理/ Fe 3 O 4(4:1)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号