当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Immuno-Matrix-Assisted Laser Desorption/Ionization Assays for Quantifying AKT1 and AKT2 in Breast and Colorectal Cancer Cell Lines and Tumors
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.analchem.7b02934 Robert Popp 1 , Huiyan Li 1 , André LeBlanc 2 , Yassene Mohammed 1, 3 , Adriana Aguilar-Mahecha 4 , Andrew G. Chambers 1 , Cathy Lan 5 , Oliver Poetz 6 , Mark Basik 5 , Gerald Batist 5 , Christoph H. Borchers 1, 2, 5, 7
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.analchem.7b02934 Robert Popp 1 , Huiyan Li 1 , André LeBlanc 2 , Yassene Mohammed 1, 3 , Adriana Aguilar-Mahecha 4 , Andrew G. Chambers 1 , Cathy Lan 5 , Oliver Poetz 6 , Mark Basik 5 , Gerald Batist 5 , Christoph H. Borchers 1, 2, 5, 7
Affiliation
|
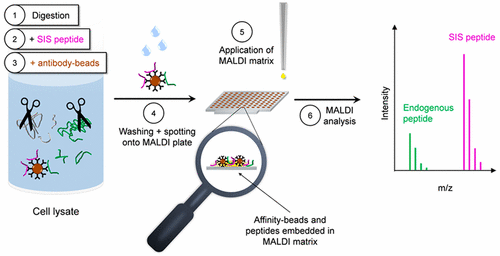
The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin (mTOR) pathway is one of the most commonly dysregulated signaling pathways that is linked to cancer development and progression, and its quantitative protein analysis holds the promise to facilitate patient stratification for targeted therapies. Whereas immunohistochemistry (IHC) and immunoassays are routinely used for clinical analysis of signaling pathways, mass spectrometry-based approaches such as liquid chromatography/electrospray ionization multiple reaction monitoring mass spectrometry (LC/ESI-MRM-MS) are more commonly used in clinical research. Both technologies have certain disadvantages, namely, the nonspecificity of IHC and immunoassays, and potentially long analysis times per sample of LC/ESI-MRM-MS. To create a robust, fast, and sensitive protein quantification tool, we developed immuno-matrix-assisted laser desorption/ionization (iMALDI) assays with automated liquid handling. The assays are able to quantify AKT1 and AKT2 from breast cancer and colon cancer cell lines and flash-frozen tumor lysates with a linear range of 0.05–2.0 fmol/μg of total lysate protein and with coefficients of variation < 15%. Compared to other mass spectrometric methods, the developed assays require less sample per analysis—only 25 μg of total protein—and are therefore suitable for analysis of needle biopsies. Furthermore, the presented iMALDI technique is the first MS-based method for absolute quantitation of AKT peptides from cancer tissues. This study demonstrates the suitability of iMALDI for low limit-of-detection and reproducible quantitation of signaling pathway members using a benchtop MALDI mass spectrometer within approximately 6–7 h.
中文翻译:

免疫基质辅助激光解吸/电离测定法定量乳腺癌和结肠直肠癌细胞系和肿瘤中的AKT1和AKT2
磷脂酰肌醇3激酶(PI3K)/蛋白激酶B(AKT)/雷帕霉素(mTOR)途径的机制靶标是与癌症发生和发展相关的最常见的信号调节途径之一,其定量蛋白质分析具有广阔的前景促进针对目标疗法的患者分层。免疫组织化学(IHC)和免疫分析通常用于信号通路的临床分析,而基于质谱的方法(例如液相色谱/电喷雾电离多反应监测质谱(LC / ESI-MRM-MS))更常用于临床研究。两种技术都有某些缺点,即IHC和免疫测定的非特异性,以及每个LC / ESI-MRM-MS样品的分析时间可能很长。为了创建一个强大,快速,和灵敏的蛋白质定量工具,我们开发了具有自动液体处理功能的免疫基质辅助激光解吸/电离(iMALDI)分析方法。该测定法能够定量分析乳腺癌和结肠癌细胞系以及速冻肿瘤裂解液中的AKT1和AKT2,其线性范围为裂解液总蛋白的0.05–2.0 fmol /μg,变异系数<15%。与其他质谱方法相比,已开发的测定方法每次分析所需的样品更少(仅25μg的总蛋白质),因此适用于针头活检的分析。此外,提出的iMALDI技术是第一种基于MS的绝对定量方法,用于从癌症组织中绝对定量AKT肽。
更新日期:2017-09-20
中文翻译:

免疫基质辅助激光解吸/电离测定法定量乳腺癌和结肠直肠癌细胞系和肿瘤中的AKT1和AKT2
磷脂酰肌醇3激酶(PI3K)/蛋白激酶B(AKT)/雷帕霉素(mTOR)途径的机制靶标是与癌症发生和发展相关的最常见的信号调节途径之一,其定量蛋白质分析具有广阔的前景促进针对目标疗法的患者分层。免疫组织化学(IHC)和免疫分析通常用于信号通路的临床分析,而基于质谱的方法(例如液相色谱/电喷雾电离多反应监测质谱(LC / ESI-MRM-MS))更常用于临床研究。两种技术都有某些缺点,即IHC和免疫测定的非特异性,以及每个LC / ESI-MRM-MS样品的分析时间可能很长。为了创建一个强大,快速,和灵敏的蛋白质定量工具,我们开发了具有自动液体处理功能的免疫基质辅助激光解吸/电离(iMALDI)分析方法。该测定法能够定量分析乳腺癌和结肠癌细胞系以及速冻肿瘤裂解液中的AKT1和AKT2,其线性范围为裂解液总蛋白的0.05–2.0 fmol /μg,变异系数<15%。与其他质谱方法相比,已开发的测定方法每次分析所需的样品更少(仅25μg的总蛋白质),因此适用于针头活检的分析。此外,提出的iMALDI技术是第一种基于MS的绝对定量方法,用于从癌症组织中绝对定量AKT肽。











































 京公网安备 11010802027423号
京公网安备 11010802027423号