当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multifunctional Glyco‐Nanofibers: siRNA Induced Supermolecular Assembly for Codelivery In Vivo
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2017-09-20 , DOI: 10.1002/adfm.201703083 Yincheng Chang 1 , Yinghua Lv 2 , Peng Wei 1 , Pengfei Zhang 2 , Liang Pu 1 , Xiaoxu Chen 2 , Kui Yang 1 , Xueliang Li 2 , Yuchao Lu 1 , Chenxi Hou 1 , Yuxin Pei 1 , Wenxian Zeng 2 , Zhichao Pei 1
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2017-09-20 , DOI: 10.1002/adfm.201703083 Yincheng Chang 1 , Yinghua Lv 2 , Peng Wei 1 , Pengfei Zhang 2 , Liang Pu 1 , Xiaoxu Chen 2 , Kui Yang 1 , Xueliang Li 2 , Yuchao Lu 1 , Chenxi Hou 1 , Yuxin Pei 1 , Wenxian Zeng 2 , Zhichao Pei 1
Affiliation
|
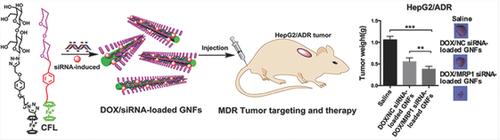
Targeted codelivery and controlled release of drug/siRNA (small interfering RNA) in a safe and effective vehicle hold great promises for overcoming drug resistance and optimal efficacy in cancer treatment; however, rational design and preparation of such vehicles remain a critical challenge. Thus, glyco‐nanofibers (GNFs) are fabricated via supermolecular assembly of polyanionic siRNA and cationic vesicles to simultaneously deliver siRNA and doxorubicin hydrochloride (DOX) in vitro and in vivo. The vesicles are created through self‐assembly of a positively charged amphiphilic lactose derivative featuring a lactose moiety and a ferrocenium unit on either end of the molecule. The GNFs display excellent biocompatibility, enhanced cell‐penetrating ability, and hepatoma targetability. The high transport efficiency of siRNA, effective gene silencing ability, and enhanced cytotoxicity to HepG2 cells of GNFs loaded with DOX are observed in vitro. Furthermore, in vivo experiments show reduced systemic toxicity and enhanced therapeutic efficacy of DOX to both HepG2 and HepG2/ADR subcutaneous tumor‐bearing nude mice. This work proves the electrostatic self‐assembly between cationic carbohydrates and polyanionic siRNA to be a convenient and effective strategy to fabricate a single vehicle for safe and effective codelivery of drug/siRNA, which can be used to combine chemo‐ and gene‐therapy against cancers and other diseases.
中文翻译:

多功能糖纳米纤维:体内siRNA诱导的超分子组装。
在安全有效的载体中靶向递送和控制药物/ siRNA(小干扰RNA)的释放有望为克服耐药性和癌症治疗的最佳疗效提供广阔前景;然而,合理设计和准备这种车辆仍然是一个严峻的挑战。因此,糖纳米纤维(GNF)是通过聚阴离子siRNA和阳离子囊泡的超分子组装而制备的,以在体外和体内同时递送siRNA和盐酸阿霉素(DOX)。囊泡是通过自组装带正电的两性乳糖衍生物而形成的,该衍生物具有一个乳糖部分和一个分子末端的二茂铁单元。GNFs具有出色的生物相容性,增强的细胞穿透能力和肝癌靶向性。siRNA的高转运效率,在体外观察到有效的基因沉默能力,以及对装有DOX的GNF的HepG2细胞增强的细胞毒性。此外,体内实验显示DOX对HepG2和HepG2 / ADR皮下荷瘤裸鼠均具有降低的全身毒性和增强的治疗效果。这项工作证明了阳离子碳水化合物和聚阴离子siRNA之间的静电自组装是一种简便有效的策略,可用于制造单一载体以安全有效地进行药物/ siRNA的代码传递,可用于结合化学疗法和基因疗法来对抗癌症和其他疾病。体内实验表明,DOX对HepG2和HepG2 / ADR皮下荷瘤裸鼠均具有降低的全身毒性和增强的治疗效果。这项工作证明了阳离子碳水化合物和聚阴离子siRNA之间的静电自组装是一种简便有效的策略,可用于制造单一载体以安全有效地进行药物/ siRNA的代码传递,可用于结合化学疗法和基因疗法来对抗癌症和其他疾病。体内实验表明,DOX对HepG2和HepG2 / ADR皮下荷瘤裸鼠均具有降低的全身毒性和增强的治疗效果。这项工作证明了阳离子碳水化合物和聚阴离子siRNA之间的静电自组装是一种简便有效的策略,可用于制造单一载体以安全有效地进行药物/ siRNA的代码传递,可用于结合化学疗法和基因疗法来对抗癌症和其他疾病。
更新日期:2017-09-20
中文翻译:

多功能糖纳米纤维:体内siRNA诱导的超分子组装。
在安全有效的载体中靶向递送和控制药物/ siRNA(小干扰RNA)的释放有望为克服耐药性和癌症治疗的最佳疗效提供广阔前景;然而,合理设计和准备这种车辆仍然是一个严峻的挑战。因此,糖纳米纤维(GNF)是通过聚阴离子siRNA和阳离子囊泡的超分子组装而制备的,以在体外和体内同时递送siRNA和盐酸阿霉素(DOX)。囊泡是通过自组装带正电的两性乳糖衍生物而形成的,该衍生物具有一个乳糖部分和一个分子末端的二茂铁单元。GNFs具有出色的生物相容性,增强的细胞穿透能力和肝癌靶向性。siRNA的高转运效率,在体外观察到有效的基因沉默能力,以及对装有DOX的GNF的HepG2细胞增强的细胞毒性。此外,体内实验显示DOX对HepG2和HepG2 / ADR皮下荷瘤裸鼠均具有降低的全身毒性和增强的治疗效果。这项工作证明了阳离子碳水化合物和聚阴离子siRNA之间的静电自组装是一种简便有效的策略,可用于制造单一载体以安全有效地进行药物/ siRNA的代码传递,可用于结合化学疗法和基因疗法来对抗癌症和其他疾病。体内实验表明,DOX对HepG2和HepG2 / ADR皮下荷瘤裸鼠均具有降低的全身毒性和增强的治疗效果。这项工作证明了阳离子碳水化合物和聚阴离子siRNA之间的静电自组装是一种简便有效的策略,可用于制造单一载体以安全有效地进行药物/ siRNA的代码传递,可用于结合化学疗法和基因疗法来对抗癌症和其他疾病。体内实验表明,DOX对HepG2和HepG2 / ADR皮下荷瘤裸鼠均具有降低的全身毒性和增强的治疗效果。这项工作证明了阳离子碳水化合物和聚阴离子siRNA之间的静电自组装是一种简便有效的策略,可用于制造单一载体以安全有效地进行药物/ siRNA的代码传递,可用于结合化学疗法和基因疗法来对抗癌症和其他疾病。



























 京公网安备 11010802027423号
京公网安备 11010802027423号