当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Universal Pattern in the Percolation and Dissipation of Protein Structural Perturbations
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.jpclett.7b02021 Nandakumar Rajasekaran 1 , Ashok Sekhar 2 , Athi N. Naganathan 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.jpclett.7b02021 Nandakumar Rajasekaran 1 , Ashok Sekhar 2 , Athi N. Naganathan 1
Affiliation
|
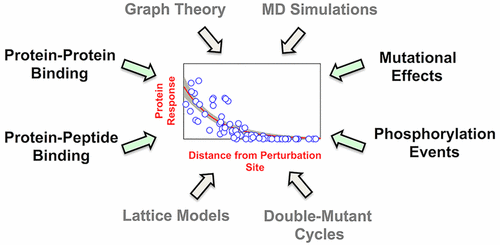
Understanding the extent to which information is transmitted through the intramolecular interaction network of proteins upon a perturbation, that is, an allosteric effect, has long remained an unsolved problem. Through an analysis of high-resolution NMR data from the literature on 28 different proteins and 49 structural perturbations, we show that the extent of induced structural changes through mutations and molecular events including protein–protein, protein–peptide, protein–ligand binding, and post-translational modifications exhibit a near-universal exponential functional form. The extent of percolation into the protein structures can be up to 20–25 Å despite no apparent change in the 3D structures. These observations are also consistent with theoretical expectations, elementary graph theoretic analysis of protein structures, detailed molecular dynamics simulations, and experimental double-mutant cycles. Our analysis highlights that most molecular events would contribute to allosteric effects independent of protein structure, topology, or identity and provides a simple avenue to test and potentially model their effects.
中文翻译:

蛋白质结构扰动的扩散和耗散的通用模式
长期以来,了解扰动通过蛋白质的分子内相互作用网络传递信息的程度(即变构效应)一直是一个尚未解决的问题。通过对来自28种不同蛋白质和49种结构扰动的文献的高分辨率NMR数据进行的分析,我们显示了通过突变和分子事件(包括蛋白质-蛋白质,蛋白质-肽,蛋白质-配体结合和翻译后修饰表现出近乎通用的指数功能形式。尽管3D结构没有明显变化,但渗入蛋白质结构的程度可高达20–25Å。这些观察结果也与理论预期,蛋白质结构的基本图理论分析,详细的分子动力学模拟和实验性双突变周期。我们的分析突出表明,大多数分子事件将独立于蛋白质结构,拓扑或同一性而导致变构作用,并提供了一种简单的方法来测试和潜在地模拟其作用。
更新日期:2017-09-20
中文翻译:

蛋白质结构扰动的扩散和耗散的通用模式
长期以来,了解扰动通过蛋白质的分子内相互作用网络传递信息的程度(即变构效应)一直是一个尚未解决的问题。通过对来自28种不同蛋白质和49种结构扰动的文献的高分辨率NMR数据进行的分析,我们显示了通过突变和分子事件(包括蛋白质-蛋白质,蛋白质-肽,蛋白质-配体结合和翻译后修饰表现出近乎通用的指数功能形式。尽管3D结构没有明显变化,但渗入蛋白质结构的程度可高达20–25Å。这些观察结果也与理论预期,蛋白质结构的基本图理论分析,详细的分子动力学模拟和实验性双突变周期。我们的分析突出表明,大多数分子事件将独立于蛋白质结构,拓扑或同一性而导致变构作用,并提供了一种简单的方法来测试和潜在地模拟其作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号