当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of Trifluoromethane Clathrate Hydrate Formation from CHF3 Gas and Ice Particles
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.jpca.7b08730 Jaruwan Amtawong 1 , Suvrajit Sengupta 1 , Michael T. Nguyen 1 , Nicole C. Carrejo 1 , Jin Guo 1 , Everly B. Fleischer 1 , Rachel W. Martin 1 , Kenneth C. Janda 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.jpca.7b08730 Jaruwan Amtawong 1 , Suvrajit Sengupta 1 , Michael T. Nguyen 1 , Nicole C. Carrejo 1 , Jin Guo 1 , Everly B. Fleischer 1 , Rachel W. Martin 1 , Kenneth C. Janda 1
Affiliation
|
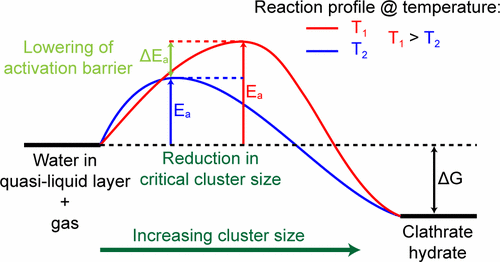
We report the formation kinetics of trifluoromethane clathrate hydrate (CH) from less than 75 μm diameter ice particles and CHF3 gas. As previously observed for difluoromethane and propane hydrate formation, the initial stages of the reaction exhibit a strong negative correlation of the reaction rate with temperature, consistent with a negative activation energy of formation. The values obtained for trifluoromethane, ca. −6 kJ/mol (H2O), are similar to those for difluoromethane, even though the two molecules have different intermolecular interactions and sizes. The activation energy is lesser per mole of H2O, but greater per mole of guest molecule, than for propane hydrate, which has a different crystal structure. We propose a possible explanation for the negative activation barrier based on the stabilization of metastable structures at low temperature. A pronounced dependence of the formation kinetics on the gas flow rate into the cell is observed. At 253 K and a flow rate of 15 mmol/h, the stage II enclathration of trifluoromethane proceeds so quickly that the overpressure, the difference between the gas cell pressure and the hydrate vapor pressure, is only 0.06 MPa.
中文翻译:

CHF 3气体和冰粒形成三氟甲烷包合物水合物的动力学
我们报告了由直径小于75μm的冰粒和CHF 3气体形成的三氟甲烷笼形水合物(CH)的形成动力学。如先前对于二氟甲烷和丙烷水合物形成所观察到的,反应的初始阶段显示出反应速率与温度的强烈负相关,与形成的负活化能一致。三氟甲烷的值,约。-6 kJ / mol(H 2 O)与二氟甲烷相似,即使两个分子之间的分子间相互作用和大小不同。每摩尔H 2的活化能较小O,但每摩尔宾客分子要比具有不同晶体结构的丙烷水合物大。我们提出了基于亚稳结构在低温下对负激活势垒的可能解释。观察到地层动力学对进入电池的气体流速有明显的依赖性。在253 K和15 mmol / h的流速下,三氟甲烷的阶段II包封反应进行得如此之快,以至于过压(气室压力与水合物蒸汽压之间的差)仅为0.06 MPa。
更新日期:2017-09-20
中文翻译:

CHF 3气体和冰粒形成三氟甲烷包合物水合物的动力学
我们报告了由直径小于75μm的冰粒和CHF 3气体形成的三氟甲烷笼形水合物(CH)的形成动力学。如先前对于二氟甲烷和丙烷水合物形成所观察到的,反应的初始阶段显示出反应速率与温度的强烈负相关,与形成的负活化能一致。三氟甲烷的值,约。-6 kJ / mol(H 2 O)与二氟甲烷相似,即使两个分子之间的分子间相互作用和大小不同。每摩尔H 2的活化能较小O,但每摩尔宾客分子要比具有不同晶体结构的丙烷水合物大。我们提出了基于亚稳结构在低温下对负激活势垒的可能解释。观察到地层动力学对进入电池的气体流速有明显的依赖性。在253 K和15 mmol / h的流速下,三氟甲烷的阶段II包封反应进行得如此之快,以至于过压(气室压力与水合物蒸汽压之间的差)仅为0.06 MPa。









































 京公网安备 11010802027423号
京公网安备 11010802027423号