当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
AgNTf2-Mediated Allylation with Allylsilanes at C3a-Position of Hexahydropyrroloindoles: Application to Total Syntheses of Amauromine Alkaloids
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.orglett.7b02602 Hiroyuki Hakamata 1 , Soichiro Sato 1 , Hirofumi Ueda 1 , Hidetoshi Tokuyama 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.orglett.7b02602 Hiroyuki Hakamata 1 , Soichiro Sato 1 , Hirofumi Ueda 1 , Hidetoshi Tokuyama 1
Affiliation
|
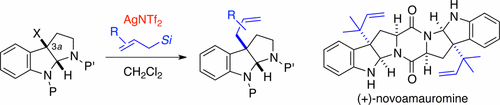
A protocol for the allylation at the C3a-position of hexahydropyrroloindole using allylsilanes is developed. AgNTf2 proved to be an efficient activator of halopyrroloindoline substrates. This method is applicable to the introduction of various allyl groups including the reverse prenyl group. The utility of this reaction is demonstrated by total synthesis of amauromine alkaloids. Stepwise bromocyclizations of the bis-indolylmethyl diketopiperazine derivative and subsequent double reverse prenylation furnished (+)-novoamauromine and (−)-epiamauromine.
中文翻译:

AgNTf 2-在C3 a处与烯丙基硅烷介导的烯丙基化-六氢吡咯并吲哚的位置:在全合成金鸟胺生物碱中的应用
一种用于在C3烯丙基化协议一个使用烯丙基硅烷hexahydropyrroloindole的位上被显影。事实证明,AgNTf 2是卤吡咯并吲哚啉底物的有效活化剂。该方法适用于引入各种烯丙基,包括反向异戊烯基。该反应的效用通过金鸟嘌呤生物碱的全合成得到证明。双吲哚甲基甲基二酮哌嗪衍生物的逐步溴环化和随后的双反向异戊烯化作用提供了(+)-新金鸟嘌呤和(-)-表金鸟嘌呤。
更新日期:2017-09-19
中文翻译:

AgNTf 2-在C3 a处与烯丙基硅烷介导的烯丙基化-六氢吡咯并吲哚的位置:在全合成金鸟胺生物碱中的应用
一种用于在C3烯丙基化协议一个使用烯丙基硅烷hexahydropyrroloindole的位上被显影。事实证明,AgNTf 2是卤吡咯并吲哚啉底物的有效活化剂。该方法适用于引入各种烯丙基,包括反向异戊烯基。该反应的效用通过金鸟嘌呤生物碱的全合成得到证明。双吲哚甲基甲基二酮哌嗪衍生物的逐步溴环化和随后的双反向异戊烯化作用提供了(+)-新金鸟嘌呤和(-)-表金鸟嘌呤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号