当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral Hypervalent Organoiodine-Catalyzed Enantioselective Oxidative Spirolactonization of Naphthol Derivatives
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.joc.7b01941 Muhammet Uyanik 1 , Takeshi Yasui 1 , Kazuaki Ishihara 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.joc.7b01941 Muhammet Uyanik 1 , Takeshi Yasui 1 , Kazuaki Ishihara 1
Affiliation
|
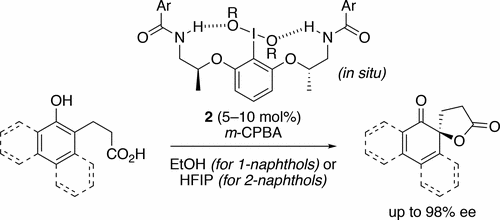
Highly enantioselective oxidative dearomatization of 2-naphthol derivatives was achieved for the first time by using conformationally flexible organoiodine catalysts derived from 2-aminoalcohol as a chiral source. Moreover, with the use of these catalysts, excellent enantioselectivities were also achieved for 1-naphthol derivatives, which had previously been obtained with only lower enantioselectivities. Furthermore, the product obtained from the present reaction could be transformed to a highly functionalized spirolactone in high yield and with excellent stereoselectivity.
中文翻译:

萘酚衍生物的手性高价有机碘催化对映选择性氧化螺电离
通过使用衍生自2-氨基醇的构象柔性有机碘催化剂作为手性来源,首次实现了2-萘酚衍生物的高度对映选择性氧化脱芳香化作用。此外,通过使用这些催化剂,对于1-萘酚衍生物也获得了优异的对映选择性,以前仅以较低的对映选择性获得了1-萘酚衍生物。此外,从本反应获得的产物可以高产率和优异的立体选择性转化为高度官能化的螺内酯。
更新日期:2017-09-19
中文翻译:

萘酚衍生物的手性高价有机碘催化对映选择性氧化螺电离
通过使用衍生自2-氨基醇的构象柔性有机碘催化剂作为手性来源,首次实现了2-萘酚衍生物的高度对映选择性氧化脱芳香化作用。此外,通过使用这些催化剂,对于1-萘酚衍生物也获得了优异的对映选择性,以前仅以较低的对映选择性获得了1-萘酚衍生物。此外,从本反应获得的产物可以高产率和优异的立体选择性转化为高度官能化的螺内酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号