当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms for C(sp2)–Si activation of aryltrimethylsilyl groups in palladium-catalysed couplings
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1039/c7ob01675a Waqar Rauf 1, 2, 3, 4 , John M. Brown 5, 6, 7, 8
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1039/c7ob01675a Waqar Rauf 1, 2, 3, 4 , John M. Brown 5, 6, 7, 8
Affiliation
|
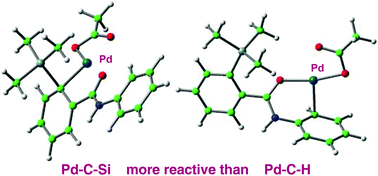
Silyl-substituted aromatic compounds can participate as the electrophilic component in palladium-catalysed cross-couplings, and reactivity is enhanced by a neighbouring silyl-group. Products analogous to those obtained from C–H activation chemistry are accessible by this means with the additional benefit of regiochemistry defined by the site of silyl substitution. DFT studies described here show that the mechanism of C–Si cleavage is distinct from previously recognised mechanisms for C–H cleavage, with a cascade of silyl intermediates en route to a stable product. The amide directing-groups are involved only in the stabilisation of palladacyclic intermediates, and are never disposed to activate silicon directly. 5-Membered and 6-membered palladacycles are known to behave differently in coupling reactions and the calculations reveal underlying reasons in the cationic pathways studied here.
中文翻译:

钯催化偶联中芳基三甲基硅烷基的C(sp 2)-Si活化机理
甲硅烷基取代的芳族化合物可以作为亲电子组分参与钯催化的交叉偶联,并且反应性通过相邻的甲硅烷基基团得到增强。通过C-H活化化学获得的类似产品可通过这种方法获得,并具有由甲硅烷基取代位点定义的区域化学的其他优点。此处描述的DFT研究表明,C–Si裂解的机理与先前公认的C–H裂解的机理不同,途中有一系列甲硅烷基中间体稳定的产品。酰胺的导向基团仅涉及到戊四环中间体的稳定化,决不直接活化硅。已知5元和6元的Palladacycles在偶联反应中的行为不同,计算结果揭示了本文研究的阳离子途径的根本原因。
更新日期:2017-09-20
中文翻译:

钯催化偶联中芳基三甲基硅烷基的C(sp 2)-Si活化机理
甲硅烷基取代的芳族化合物可以作为亲电子组分参与钯催化的交叉偶联,并且反应性通过相邻的甲硅烷基基团得到增强。通过C-H活化化学获得的类似产品可通过这种方法获得,并具有由甲硅烷基取代位点定义的区域化学的其他优点。此处描述的DFT研究表明,C–Si裂解的机理与先前公认的C–H裂解的机理不同,途中有一系列甲硅烷基中间体稳定的产品。酰胺的导向基团仅涉及到戊四环中间体的稳定化,决不直接活化硅。已知5元和6元的Palladacycles在偶联反应中的行为不同,计算结果揭示了本文研究的阳离子途径的根本原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号