当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An expedient synthesis of flexible nucleosides via a regiocontrolled enzymatic glycosylation of functionalized imidazoles
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1039/c7ob01850a S. Vichier-Guerre 1, 2, 3, 4, 5 , L. Dugué 1, 2, 3, 4, 5 , F. Bonhomme 1, 2, 3, 4, 5 , S. Pochet 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1039/c7ob01850a S. Vichier-Guerre 1, 2, 3, 4, 5 , L. Dugué 1, 2, 3, 4, 5 , F. Bonhomme 1, 2, 3, 4, 5 , S. Pochet 1, 2, 3, 4, 5
Affiliation
|
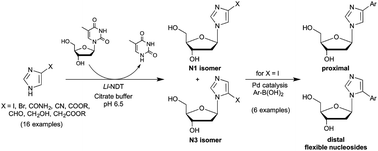
A versatile two-step synthesis of C4- and C5-arylated 2′-deoxyribosylimidazoles was elaborated using enzymatic N-transglycosylation followed by microwave-assisted Pd-catalysed arylation reactions. We report herein the reaction conditions that permit managing regioselectivity (N3 versus N1-isomers) in the enzymatic glycosylation of 4-iodoimidazole using the nucleoside N-deoxyribosyltransferase from L. leichmannii. Regiocontrolled glycosylation was also observed among several other imidazole derivatives studied, providing simple access to isomers not readily accessible by chemical routes. Finally, a series of flexible nucleosides was obtained in one step from 4- or 5-iodo-imidazole nucleosides by the Suzuki–Miyaura cross-coupling reaction with (hetero)aryl-boronic acids in aqueous media. Moreover, this chemoenzymatic approach is compatible with a one-pot two-step process affording a straightforward access to a broad array of potential anticancer and antiviral drugs as well as new DNA building blocks.
中文翻译:

通过功能化的咪唑的区域控制酶促糖基化来合成柔性核苷
C4-和C5-芳基化的2'-脱氧核糖基咪唑的多功能两步合成方法是使用酶促N-转糖基化,然后进行微波辅助的Pd催化的芳基化反应。我们在此报告了反应条件,该条件允许使用莱希曼氏乳杆菌的核苷N-脱氧核糖基转移酶处理4-碘咪唑的酶促糖基化反应中的区域选择性(N3对N1-异构体)。在其他研究的其他咪唑衍生物中也观察到了区域控制的糖基化作用,从而提供了通过化学途径不易获得的异构体的简便途径。最后,一步一步从4-或5-碘-咪唑核苷通过Suzuki-Miyaura与(杂)芳基-硼酸在水性介质中的交叉偶联反应一步获得了一系列柔性核苷。此外,这种化学酶法与一锅两步法兼容,可直接获得各种潜在的抗癌和抗病毒药物以及新的DNA构建基块。
更新日期:2017-09-20
中文翻译:

通过功能化的咪唑的区域控制酶促糖基化来合成柔性核苷
C4-和C5-芳基化的2'-脱氧核糖基咪唑的多功能两步合成方法是使用酶促N-转糖基化,然后进行微波辅助的Pd催化的芳基化反应。我们在此报告了反应条件,该条件允许使用莱希曼氏乳杆菌的核苷N-脱氧核糖基转移酶处理4-碘咪唑的酶促糖基化反应中的区域选择性(N3对N1-异构体)。在其他研究的其他咪唑衍生物中也观察到了区域控制的糖基化作用,从而提供了通过化学途径不易获得的异构体的简便途径。最后,一步一步从4-或5-碘-咪唑核苷通过Suzuki-Miyaura与(杂)芳基-硼酸在水性介质中的交叉偶联反应一步获得了一系列柔性核苷。此外,这种化学酶法与一锅两步法兼容,可直接获得各种潜在的抗癌和抗病毒药物以及新的DNA构建基块。



























 京公网安备 11010802027423号
京公网安备 11010802027423号