当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning linkage isomerism and magnetic properties of bi- and tri-metallic cage silsesquioxanes by cation and solvent effects
Dalton Transactions ( IF 3.5 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1039/c7dt02017a Alexey N. Bilyachenko 1, 2, 3, 4, 5 , Alexander A. Korlyukov 1, 2, 3, 6, 7 , Anna V. Vologzhanina 1, 2, 3 , Victor N. Khrustalev 3, 4, 5 , Alena N. Kulakova 1, 2, 3 , Jérôme Long 8, 9, 10, 11, 12 , Joulia Larionova 8, 9, 10, 11, 12 , Yannick Guari 8, 9, 10, 11, 12 , Marina S. Dronova 1, 2, 3 , Ulyana S. Tsareva 1, 2, 3 , Pavel V. Dorovatovskii 3, 13, 14 , Elena S. Shubina 1, 2, 3 , Mikhail M. Levitsky 1, 2, 3
Dalton Transactions ( IF 3.5 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1039/c7dt02017a Alexey N. Bilyachenko 1, 2, 3, 4, 5 , Alexander A. Korlyukov 1, 2, 3, 6, 7 , Anna V. Vologzhanina 1, 2, 3 , Victor N. Khrustalev 3, 4, 5 , Alena N. Kulakova 1, 2, 3 , Jérôme Long 8, 9, 10, 11, 12 , Joulia Larionova 8, 9, 10, 11, 12 , Yannick Guari 8, 9, 10, 11, 12 , Marina S. Dronova 1, 2, 3 , Ulyana S. Tsareva 1, 2, 3 , Pavel V. Dorovatovskii 3, 13, 14 , Elena S. Shubina 1, 2, 3 , Mikhail M. Levitsky 1, 2, 3
Affiliation
|
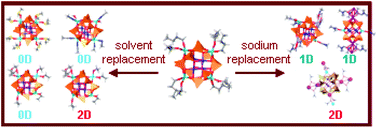
Herein, the effect of replacement of the surrounding solvent and/or the partial substitution of sodium ions in the cage-like copper, sodium phenylsilsesquioxane [(PhSiO1.5)12(CuO)4(NaO0.5)4(n-BuOH)6] 1 with a globular structure was investigated; the synthesis of ten new derivatives of complex 1 was performed, and their crystal structures were determined. Solvate replacement of n-butanol in 1 with dimethyl sulfoxide, acetonitrile, 1,4-dioxane/EtOH, and 1,4-dioxane/PhCN afforded the complexes [(PhSiO1.5)12(CuO)4(NaO0.5)4(DMSO)8] (2), [(PhSiO1.5)12(CuO)4(NaO0.5)4(MeCN)6(H2O)2]·2MeCN (3), [(PhSiO1.5)12(CuO)4(NaO0.5)4(C4H8O2)(EtOH)4]·0.5EtOH (4), and [(PhSiO1.5)12(CuO)4(NaO0.5)4(C4H8O2)4(H2O)3]·2PhCN·2C4H8O2·H2O (5). In an aqueous solution of EtOH, a rearrangement reaction occurred instead of substitution, which resulted in a new complex, [(PhSiO1.5)10(CuO)2(NaO0.5)2(H2O)6] (6), with a cooling tower molecular structure. Transmetalation reactions of 1 with KCl or CsF resulted in the formation of the trimetallic complexes [(PhSiO1.5)12(CuO)4(NaO0.5)2(KO0.5)2(DMF)6] (7), [(PhSiO1.5)12(CuO)4(NaO0.5)(CsO0.5)3(DMF)4(DMSO)(H2O)]·1.5DMF (8), [(PhSiO1.5)12(CuO)4(NaO0.5)2(CsO0.5)2(DMF)8]·0.5H2O (9), and [(PhSiO1.5)12(CuO)4(NaO0.5)3(CsO0.5)(DMF)4] (10). The replacement of sodium ions of 1 by the bulky non-metallic cation PhMe3N+ upon the interaction of 1 with PhMe3NCl in a MeCN medium afforded the complex (PhNMe3)2[(PhSiO1.5)12(CuO)4(NaO0.5)2(O)(MeCN)4]·4MeCN (11). X-ray analysis confirmed the successful replacement of terminal butanol molecules in all complexes and the stability of the globular cage of the parent complex under the reaction conditions, with the exceptions of complexes 6 and 9 that underwent an unprecedented reorganization of cage metallasilsesquioxane (CLMS) units into the cooling tower and sandwich-like cages, respectively. The stability of the cage during substitution reactions provides the first experimental evidence that polynuclear cage metallasilsesquioxanes can act as building blocks for the construction of coordination polymers, opening new ways for the synthesis of hybrid CLMS-based frameworks. In particular, the compounds 4, 6, 7, 9, and 10 are one-dimensional coordination polymers, and 5 and 8 are two-dimensional coordination polymers with a square lattice topology. A magnetism study of the coordination polymer compounds 5, 6, 8, and 9 showed antiferromagnetic behavior between copper centers.
中文翻译:

通过阳离子和溶剂效应调节双金属和三金属笼型倍半硅氧烷的键合异构性和磁性
在此,笼型铜苯基倍半硅氧烷钠[(PhSiO 1.5)12(CuO)4(NaO 0.5)4(n -BuOH)6 ]中周围溶剂的替代和/或钠离子的部分替代的效果。研究了具有球形结构的1个;合成了十种新的配合物1的衍生物,并确定了它们的晶体结构。溶剂化物置换Ñ丁醇中1与二甲亚砜,乙腈,1,4-二恶烷/乙醇,1,4-二恶烷/ PHCN得到配合物[(PhSiO 1.5)12(CuO)4(NaO 0.5)4(DMSO)8 ](2),[(PhSiO 1.5)12(CuO)4(NaO 0.5)4(MeCN)6(H 2 O)2 ]·2MeCN(3), [(PhSiO 1.5)12(CuO)4(NaO 0.5)4(C 4 H 8 O 2)(EtOH)4 ]·0.5EtOH(4)和[(PhSiO 1.5)12(CuO)4(NaO 0.5) 4(C 4 H 8 O 2) 4(H 2 O) 3 ]·2PhCN·2C 4 H 8 O 2 ·H 2 O( 5)。在EtOH水溶液中,发生了重排反应而不是取代反应,从而产生了新的络合物[[PhSiO 1.5) 10(CuO) 2(NaO 0.5) 2(H 2 O) 6 ]( 6),冷却塔的分子结构。的重金属化反应1用KCl或CsF导致形成三金属配合物[(PhSiO 1.5)12(CuO)4(NaO 0.5)2(KO 0.5)2(DMF)6 ](7),[(PhSiO 1.5)12(CuO )4(NaO 0.5)(CsO 0.5)3(DMF)4(DMSO)(H 2 O)]·1.5DMF(8),[(PhSiO 1.5)12(CuO)4(NaO 0.5)2(CsO0.5) 2(DMF) 8 ]·0.5H 2 O( 9),和[(PhSiO 1.5) 12(CuO) 4(NaO 0.5) 3(CsO 0.5)(DMF) 4 ]( 10)。在MeCN介质中1与PhMe 3 NCl相互作用后,笨重的非金属阳离子PhMe 3 N +代替钠离子1生成了复合物(PhNMe 3) 2 [(PhSiO 1.5) 12(CuO) 4(NaO 0.5)2(O)(MeCN)4 ]·4MeCN(11)。X射线分析证实,在反应条件下,除了配合物6和9之外,所有配合物中末端丁醇分子均能成功替换,并且母体配合物的球状笼具有稳定性。对笼式金属硅倍半氧烷(CLMS)单元分别进行了前所未有的重组,分别放入冷却塔和类似三明治的笼子中。笼子在取代反应过程中的稳定性提供了第一个实验证据,表明多核笼子金属硅倍半氧烷可以作为配位聚合物构建的基石,为合成基于CLMS的混合骨架开辟了新途径。特别是,化合物4,6,7,9,和10是一维的配位聚合物,和5和8是具有正方形晶格拓扑的二维配位聚合物。该配位聚合物的磁性研究化合物5,6,8,和9表明铜中心之间的反铁磁行为。
更新日期:2017-09-20
中文翻译:

通过阳离子和溶剂效应调节双金属和三金属笼型倍半硅氧烷的键合异构性和磁性
在此,笼型铜苯基倍半硅氧烷钠[(PhSiO 1.5)12(CuO)4(NaO 0.5)4(n -BuOH)6 ]中周围溶剂的替代和/或钠离子的部分替代的效果。研究了具有球形结构的1个;合成了十种新的配合物1的衍生物,并确定了它们的晶体结构。溶剂化物置换Ñ丁醇中1与二甲亚砜,乙腈,1,4-二恶烷/乙醇,1,4-二恶烷/ PHCN得到配合物[(PhSiO 1.5)12(CuO)4(NaO 0.5)4(DMSO)8 ](2),[(PhSiO 1.5)12(CuO)4(NaO 0.5)4(MeCN)6(H 2 O)2 ]·2MeCN(3), [(PhSiO 1.5)12(CuO)4(NaO 0.5)4(C 4 H 8 O 2)(EtOH)4 ]·0.5EtOH(4)和[(PhSiO 1.5)12(CuO)4(NaO 0.5) 4(C 4 H 8 O 2) 4(H 2 O) 3 ]·2PhCN·2C 4 H 8 O 2 ·H 2 O( 5)。在EtOH水溶液中,发生了重排反应而不是取代反应,从而产生了新的络合物[[PhSiO 1.5) 10(CuO) 2(NaO 0.5) 2(H 2 O) 6 ]( 6),冷却塔的分子结构。的重金属化反应1用KCl或CsF导致形成三金属配合物[(PhSiO 1.5)12(CuO)4(NaO 0.5)2(KO 0.5)2(DMF)6 ](7),[(PhSiO 1.5)12(CuO )4(NaO 0.5)(CsO 0.5)3(DMF)4(DMSO)(H 2 O)]·1.5DMF(8),[(PhSiO 1.5)12(CuO)4(NaO 0.5)2(CsO0.5) 2(DMF) 8 ]·0.5H 2 O( 9),和[(PhSiO 1.5) 12(CuO) 4(NaO 0.5) 3(CsO 0.5)(DMF) 4 ]( 10)。在MeCN介质中1与PhMe 3 NCl相互作用后,笨重的非金属阳离子PhMe 3 N +代替钠离子1生成了复合物(PhNMe 3) 2 [(PhSiO 1.5) 12(CuO) 4(NaO 0.5)2(O)(MeCN)4 ]·4MeCN(11)。X射线分析证实,在反应条件下,除了配合物6和9之外,所有配合物中末端丁醇分子均能成功替换,并且母体配合物的球状笼具有稳定性。对笼式金属硅倍半氧烷(CLMS)单元分别进行了前所未有的重组,分别放入冷却塔和类似三明治的笼子中。笼子在取代反应过程中的稳定性提供了第一个实验证据,表明多核笼子金属硅倍半氧烷可以作为配位聚合物构建的基石,为合成基于CLMS的混合骨架开辟了新途径。特别是,化合物4,6,7,9,和10是一维的配位聚合物,和5和8是具有正方形晶格拓扑的二维配位聚合物。该配位聚合物的磁性研究化合物5,6,8,和9表明铜中心之间的反铁磁行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号