当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile activation of alkynes with a boraguanidinato-stabilized germylene: a combined experimental and theoretical study
Dalton Transactions ( IF 3.5 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1039/c7dt01950e Jiří Böserle 1, 2, 3, 4, 5 , Grigory Zhigulin 6, 7, 8 , Petr Štěpnička 5, 9, 10, 11, 12 , Filip Horký 5, 9, 10, 11, 12 , Milan Erben 1, 2, 3, 4, 5 , Roman Jambor 1, 2, 3, 4, 5 , Aleš Růžička 1, 2, 3, 4, 5 , Sergey Ketkov 6, 7, 8 , Libor Dostál 1, 2, 3, 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1039/c7dt01950e Jiří Böserle 1, 2, 3, 4, 5 , Grigory Zhigulin 6, 7, 8 , Petr Štěpnička 5, 9, 10, 11, 12 , Filip Horký 5, 9, 10, 11, 12 , Milan Erben 1, 2, 3, 4, 5 , Roman Jambor 1, 2, 3, 4, 5 , Aleš Růžička 1, 2, 3, 4, 5 , Sergey Ketkov 6, 7, 8 , Libor Dostál 1, 2, 3, 4, 5
Affiliation
|
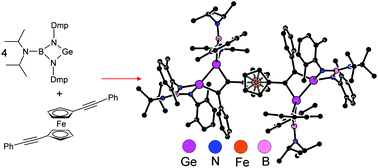
A boraguanidinato-stabilized germylene, [(i-Pr)2NB(N-2,6-Me2C6H3)2]Ge, reacts with alkynes RC![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) CR selectively in a 2
CR selectively in a 2 :
: 1 molar ratio to afford 3,4-R,R′-1,2-digermacyclobut-3-enes 1a–e as the products of formal [2 + 2 + 2] cyclization [R/R′ = Me/Me (1a), Ph/Ph (1b), Ph/H (1c), t-Bu/H (1d) and Cy/H (1e)]. Ferrocenyl-substituted alkynes react similarly, yielding the corresponding ferrocenylated 3,4-R,R′-1,2-digermacyclobut-3-enes 2a–d [where R/R′ = Fc/H (2a), Fc/Me (2b), Fc/Ph (2c), and Fc/Fc (2d); Fc = ferrocenyl]. By contrast, only one of the triple bonds available in conjugated diynes RC
1 molar ratio to afford 3,4-R,R′-1,2-digermacyclobut-3-enes 1a–e as the products of formal [2 + 2 + 2] cyclization [R/R′ = Me/Me (1a), Ph/Ph (1b), Ph/H (1c), t-Bu/H (1d) and Cy/H (1e)]. Ferrocenyl-substituted alkynes react similarly, yielding the corresponding ferrocenylated 3,4-R,R′-1,2-digermacyclobut-3-enes 2a–d [where R/R′ = Fc/H (2a), Fc/Me (2b), Fc/Ph (2c), and Fc/Fc (2d); Fc = ferrocenyl]. By contrast, only one of the triple bonds available in conjugated diynes RC![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) CC
CC![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) CR is activated with the germylene, while the second one remains intact even in the presence of an excess of the germylene. The exclusive formation of 3,4-R,(C
CR is activated with the germylene, while the second one remains intact even in the presence of an excess of the germylene. The exclusive formation of 3,4-R,(C![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) CR)-1,2-digermacyclobut-3-enes 3a–c [R = Ph (3a), t-Bu(3b), and Fc (3c)] was ascribed to a steric repulsion around the second triple bond. On the other hand, the reaction of the germylene with more flexible dialkyne fc(C
CR)-1,2-digermacyclobut-3-enes 3a–c [R = Ph (3a), t-Bu(3b), and Fc (3c)] was ascribed to a steric repulsion around the second triple bond. On the other hand, the reaction of the germylene with more flexible dialkyne fc(C![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) CPh)2 (fc = ferrocene-1,1′-diyl) proceeded in the expected manner, producing compound 4, where both triple bonds are transformed into 1,2-digermacyclobut-3-ene rings by reaction with four equivalents of the germylene. All compounds were characterized by multinuclear NMR spectroscopy, Raman and IR spectroscopy, and in the case of 1a–c, 2a, 2c, 3a, 3b and 4, also by single-crystal X-ray diffraction analysis. The ferrocenyl substituted compounds were studied by cyclic voltammetry (CV). Finally, the plausible reaction pathway was studied for a model reaction of [(i-Pr)2NB(N-2,6-Me2C6H3)2]Ge with MeC
CPh)2 (fc = ferrocene-1,1′-diyl) proceeded in the expected manner, producing compound 4, where both triple bonds are transformed into 1,2-digermacyclobut-3-ene rings by reaction with four equivalents of the germylene. All compounds were characterized by multinuclear NMR spectroscopy, Raman and IR spectroscopy, and in the case of 1a–c, 2a, 2c, 3a, 3b and 4, also by single-crystal X-ray diffraction analysis. The ferrocenyl substituted compounds were studied by cyclic voltammetry (CV). Finally, the plausible reaction pathway was studied for a model reaction of [(i-Pr)2NB(N-2,6-Me2C6H3)2]Ge with MeC![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) CMe using DFT computations.
CMe using DFT computations.
中文翻译:

硼烷胍基稳定的亚二甲基苯易于活化炔烃:组合的实验和理论研究
硼胍基稳定的二甲苯,[[(i-Pr)2 NB(N -2,6-Me 2 C 6 H 3)2 ] Ge,与炔烃RC![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) CR选择性地以2
CR选择性地以2  :
: 1的摩尔比反应,得到3,4 -R,R'-1,2-二甲基环丁-3-烯1a–e作为正式[2 + 2 + 2]环化的产物[R / R'= Me / Me(1a),Ph / Ph(1b) ,Ph / H(1c),t -Bu / H(1d)和Cy / H(1e)]。二茂铁基取代的炔烃反应类似,得到相应的二茂铁基化的3,4-R,R'-1,2-二茂铁环丁-3-烯2a–d[其中R / R′= Fc / H(2a),Fc / Me(2b),Fc / Ph(2c)和Fc / Fc(2d);Fc =二茂铁基]。相比之下,共轭二炔RC
1的摩尔比反应,得到3,4 -R,R'-1,2-二甲基环丁-3-烯1a–e作为正式[2 + 2 + 2]环化的产物[R / R'= Me / Me(1a),Ph / Ph(1b) ,Ph / H(1c),t -Bu / H(1d)和Cy / H(1e)]。二茂铁基取代的炔烃反应类似,得到相应的二茂铁基化的3,4-R,R'-1,2-二茂铁环丁-3-烯2a–d[其中R / R′= Fc / H(2a),Fc / Me(2b),Fc / Ph(2c)和Fc / Fc(2d);Fc =二茂铁基]。相比之下,共轭二炔RC ![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) CC
CC ![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) CR中可用的三键中只有一个被亚二甲基激活,而第二个即使在亚甲基二甲苯过量的情况下也保持完整。
CR中可用的三键中只有一个被亚二甲基激活,而第二个即使在亚甲基二甲苯过量的情况下也保持完整。![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) 归因于3,4-R,(C CR)-1,2-二茂铁环丁-3-烯3a–c [R = Ph(3a),t -Bu(3b)和Fc(3c)]的排他性形成。第二个三键周围的空间排斥。另一方面,亚二甲基与更灵活的二炔fc(C
归因于3,4-R,(C CR)-1,2-二茂铁环丁-3-烯3a–c [R = Ph(3a),t -Bu(3b)和Fc(3c)]的排他性形成。第二个三键周围的空间排斥。另一方面,亚二甲基与更灵活的二炔fc(C ![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) CPh)2的反应(fc =二茂铁-1,1′-二基)以预期的方式进行,产生化合物4,其中两个三键通过与四当量的亚二甲苯基反应而转化成1,2-二茂铁环丁-3-烯环。所有化合物均通过多核NMR光谱,拉曼光谱和IR光谱进行表征,在1a–c,2a,2c,3a,3b和4的情况下,还通过单晶X射线衍射分析进行表征。通过循环伏安法(CV)研究了二茂铁基取代的化合物。最后,研究了[[i-Pr)2 NB(N -2,6-Me 2 C6 H 3) 2 ] Ge与MeC
CPh)2的反应(fc =二茂铁-1,1′-二基)以预期的方式进行,产生化合物4,其中两个三键通过与四当量的亚二甲苯基反应而转化成1,2-二茂铁环丁-3-烯环。所有化合物均通过多核NMR光谱,拉曼光谱和IR光谱进行表征,在1a–c,2a,2c,3a,3b和4的情况下,还通过单晶X射线衍射分析进行表征。通过循环伏安法(CV)研究了二茂铁基取代的化合物。最后,研究了[[i-Pr)2 NB(N -2,6-Me 2 C6 H 3) 2 ] Ge与MeC![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) CMe一起使用DFT计算。
CMe一起使用DFT计算。
更新日期:2017-09-20
![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) CR selectively in a 2
CR selectively in a 2 :
: 1 molar ratio to afford 3,4-R,R′-1,2-digermacyclobut-3-enes 1a–e as the products of formal [2 + 2 + 2] cyclization [R/R′ = Me/Me (1a), Ph/Ph (1b), Ph/H (1c), t-Bu/H (1d) and Cy/H (1e)]. Ferrocenyl-substituted alkynes react similarly, yielding the corresponding ferrocenylated 3,4-R,R′-1,2-digermacyclobut-3-enes 2a–d [where R/R′ = Fc/H (2a), Fc/Me (2b), Fc/Ph (2c), and Fc/Fc (2d); Fc = ferrocenyl]. By contrast, only one of the triple bonds available in conjugated diynes RC
1 molar ratio to afford 3,4-R,R′-1,2-digermacyclobut-3-enes 1a–e as the products of formal [2 + 2 + 2] cyclization [R/R′ = Me/Me (1a), Ph/Ph (1b), Ph/H (1c), t-Bu/H (1d) and Cy/H (1e)]. Ferrocenyl-substituted alkynes react similarly, yielding the corresponding ferrocenylated 3,4-R,R′-1,2-digermacyclobut-3-enes 2a–d [where R/R′ = Fc/H (2a), Fc/Me (2b), Fc/Ph (2c), and Fc/Fc (2d); Fc = ferrocenyl]. By contrast, only one of the triple bonds available in conjugated diynes RC![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) CC
CC![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) CR is activated with the germylene, while the second one remains intact even in the presence of an excess of the germylene. The exclusive formation of 3,4-R,(C
CR is activated with the germylene, while the second one remains intact even in the presence of an excess of the germylene. The exclusive formation of 3,4-R,(C![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) CR)-1,2-digermacyclobut-3-enes 3a–c [R = Ph (3a), t-Bu(3b), and Fc (3c)] was ascribed to a steric repulsion around the second triple bond. On the other hand, the reaction of the germylene with more flexible dialkyne fc(C
CR)-1,2-digermacyclobut-3-enes 3a–c [R = Ph (3a), t-Bu(3b), and Fc (3c)] was ascribed to a steric repulsion around the second triple bond. On the other hand, the reaction of the germylene with more flexible dialkyne fc(C![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) CPh)2 (fc = ferrocene-1,1′-diyl) proceeded in the expected manner, producing compound 4, where both triple bonds are transformed into 1,2-digermacyclobut-3-ene rings by reaction with four equivalents of the germylene. All compounds were characterized by multinuclear NMR spectroscopy, Raman and IR spectroscopy, and in the case of 1a–c, 2a, 2c, 3a, 3b and 4, also by single-crystal X-ray diffraction analysis. The ferrocenyl substituted compounds were studied by cyclic voltammetry (CV). Finally, the plausible reaction pathway was studied for a model reaction of [(i-Pr)2NB(N-2,6-Me2C6H3)2]Ge with MeC
CPh)2 (fc = ferrocene-1,1′-diyl) proceeded in the expected manner, producing compound 4, where both triple bonds are transformed into 1,2-digermacyclobut-3-ene rings by reaction with four equivalents of the germylene. All compounds were characterized by multinuclear NMR spectroscopy, Raman and IR spectroscopy, and in the case of 1a–c, 2a, 2c, 3a, 3b and 4, also by single-crystal X-ray diffraction analysis. The ferrocenyl substituted compounds were studied by cyclic voltammetry (CV). Finally, the plausible reaction pathway was studied for a model reaction of [(i-Pr)2NB(N-2,6-Me2C6H3)2]Ge with MeC![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) CMe using DFT computations.
CMe using DFT computations.
中文翻译:

硼烷胍基稳定的亚二甲基苯易于活化炔烃:组合的实验和理论研究
硼胍基稳定的二甲苯,[[(i-Pr)2 NB(N -2,6-Me 2 C 6 H 3)2 ] Ge,与炔烃RC
![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) CR选择性地以2
CR选择性地以2  :
: 1的摩尔比反应,得到3,4 -R,R'-1,2-二甲基环丁-3-烯1a–e作为正式[2 + 2 + 2]环化的产物[R / R'= Me / Me(1a),Ph / Ph(1b) ,Ph / H(1c),t -Bu / H(1d)和Cy / H(1e)]。二茂铁基取代的炔烃反应类似,得到相应的二茂铁基化的3,4-R,R'-1,2-二茂铁环丁-3-烯2a–d[其中R / R′= Fc / H(2a),Fc / Me(2b),Fc / Ph(2c)和Fc / Fc(2d);Fc =二茂铁基]。相比之下,共轭二炔RC
1的摩尔比反应,得到3,4 -R,R'-1,2-二甲基环丁-3-烯1a–e作为正式[2 + 2 + 2]环化的产物[R / R'= Me / Me(1a),Ph / Ph(1b) ,Ph / H(1c),t -Bu / H(1d)和Cy / H(1e)]。二茂铁基取代的炔烃反应类似,得到相应的二茂铁基化的3,4-R,R'-1,2-二茂铁环丁-3-烯2a–d[其中R / R′= Fc / H(2a),Fc / Me(2b),Fc / Ph(2c)和Fc / Fc(2d);Fc =二茂铁基]。相比之下,共轭二炔RC ![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) CC
CC ![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) CR中可用的三键中只有一个被亚二甲基激活,而第二个即使在亚甲基二甲苯过量的情况下也保持完整。
CR中可用的三键中只有一个被亚二甲基激活,而第二个即使在亚甲基二甲苯过量的情况下也保持完整。![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) 归因于3,4-R,(C CR)-1,2-二茂铁环丁-3-烯3a–c [R = Ph(3a),t -Bu(3b)和Fc(3c)]的排他性形成。第二个三键周围的空间排斥。另一方面,亚二甲基与更灵活的二炔fc(C
归因于3,4-R,(C CR)-1,2-二茂铁环丁-3-烯3a–c [R = Ph(3a),t -Bu(3b)和Fc(3c)]的排他性形成。第二个三键周围的空间排斥。另一方面,亚二甲基与更灵活的二炔fc(C ![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) CPh)2的反应(fc =二茂铁-1,1′-二基)以预期的方式进行,产生化合物4,其中两个三键通过与四当量的亚二甲苯基反应而转化成1,2-二茂铁环丁-3-烯环。所有化合物均通过多核NMR光谱,拉曼光谱和IR光谱进行表征,在1a–c,2a,2c,3a,3b和4的情况下,还通过单晶X射线衍射分析进行表征。通过循环伏安法(CV)研究了二茂铁基取代的化合物。最后,研究了[[i-Pr)2 NB(N -2,6-Me 2 C6 H 3) 2 ] Ge与MeC
CPh)2的反应(fc =二茂铁-1,1′-二基)以预期的方式进行,产生化合物4,其中两个三键通过与四当量的亚二甲苯基反应而转化成1,2-二茂铁环丁-3-烯环。所有化合物均通过多核NMR光谱,拉曼光谱和IR光谱进行表征,在1a–c,2a,2c,3a,3b和4的情况下,还通过单晶X射线衍射分析进行表征。通过循环伏安法(CV)研究了二茂铁基取代的化合物。最后,研究了[[i-Pr)2 NB(N -2,6-Me 2 C6 H 3) 2 ] Ge与MeC![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) CMe一起使用DFT计算。
CMe一起使用DFT计算。











































 京公网安备 11010802027423号
京公网安备 11010802027423号