当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, characterisation and in vitro cytotoxicity of mixed ligand Pt(II) oxadiazoline complexes with hexamethylenetetramine and 7-nitro-1,3,5-triazaadamantane
Dalton Transactions ( IF 3.5 ) Pub Date : 2017-08-21 00:00:00 , DOI: 10.1039/c7dt02406a Stefanie Sieste 1, 2, 3, 4 , Irina Lifincev 1, 2, 3, 4 , Nina Stein 1, 2, 3, 4 , Gabriele Wagner 1, 2, 3, 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2017-08-21 00:00:00 , DOI: 10.1039/c7dt02406a Stefanie Sieste 1, 2, 3, 4 , Irina Lifincev 1, 2, 3, 4 , Nina Stein 1, 2, 3, 4 , Gabriele Wagner 1, 2, 3, 4, 5
Affiliation
|
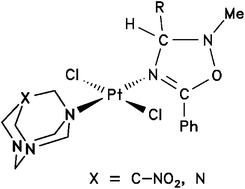
trans-Platinum(II) oxadiazoline complexes with 7-nitro-1,3,5-triazaadamantane (NO2-TAA) or hexamethylenetetramine (hmta) ligands have been synthesised from trans-[PtCl2(PhCN)2] via cycloaddition of nitrones to one of the coordinated nitriles, followed by exchange of the other nitrile by NO2-TAA or hmta. Stoichiometric control allows for the selective synthesis of mono- and dinuclear complexes where 7-NO2TAA and hmta act as mono- and bidentate ligands, respectively. Precursors and the target complexes trans-[PtCl2(hmta)(oxadiazoline)], trans-[PtCl2(NO2-TAA)(oxadiazoline)] and trans-[{PtCl2(oxadiazoline)}2(hmta)] were characterised by elemental analysis, IR and multinuclear (1H, 13C, 195Pt) NMR spectroscopy. DFT (B3LYP/6-31G*/LANL08) and AIM calculations suggest a stronger bonding of hmta with the [PtCl2(oxadiazoline)] fragment, in agreement with the experimentally observed reactivity in the ligand exchange (hmta > 7-NO2TAA). Replacement of the nitrile by hmta is predicted to be more exothermic than that with 7-NO2-TAA, although the activation barriers are similar. Protonation of the non-coordinated N atoms is anticipated to weaken the Pt–N bond and lower the activation barrier for ligand exchange. This effect might help activate these compounds in a slightly acidic environment such as some tumour tissues. Ten of the new compounds were tested for their in vitro cytotoxicity in the human cancer cell lines HeLa and A549. Some of the mononuclear complexes are more potent than cisplatin, and their activity is still high in A549 where cisplatin shows little effect. The dinuclear complexes are inactive, presumably due to their lipophilicity and reduced solubility in water.
中文翻译:

六亚甲基四胺与7-硝基-1,3,5-三氮杂金刚烷混合配体Pt(II)恶二唑啉配合物的合成,表征及体外细胞毒性
反式-铂(II)配合物恶二唑啉用7-硝基-1,3,5- triazaadamantane(NO 2 -TAA)或六亚甲基四胺(HMTA)配位体已经从合成反式- [氯铂酸2(PHCN)2 ]通过硝酮的环加成到一个配位的腈中,然后用NO 2 -TAA或hmta交换另一个腈。化学计量控制允许选择性合成单核和双核配合物,其中7-NO 2 TAA和hmta分别充当单和二齿配体。前体和目标配合物反式-[PtCl 2(hmta)(恶二唑啉)],反式-[PtCl 2(NO 2 -TAA)(恶二唑啉)]和反式-[{PtCl 2(恶二唑啉)} 2(hmta)]的元素分析,IR和多核(1 H,13 C,195 Pt)NMR表征光谱学。DFT(B3LYP / 6-31G * / LANL08)和AIM计算表明hmta与[PtCl 2(恶二唑啉)]片段的结合更牢固,与配体交换中实验观察到的反应性一致(hmta> 7-NO 2 TAA )。预计用hmta代替腈会比用7-NO 2放热更多。-TAA,尽管激活障碍相似。预期非配位的N原子的质子化会削弱Pt–N键并降低配体交换的激活势垒。这种作用可能有助于在弱酸性环境(例如某些肿瘤组织)中激活这些化合物。测试了十种新化合物在人癌细胞系HeLa和A549中的体外细胞毒性。一些单核复合物比顺铂更有效,并且它们的活性在A549中仍然很高,而顺铂几乎没有作用。双核复合物是无活性的,大概是由于它们的亲脂性和在水中的溶解度降低。
更新日期:2017-09-20
中文翻译:

六亚甲基四胺与7-硝基-1,3,5-三氮杂金刚烷混合配体Pt(II)恶二唑啉配合物的合成,表征及体外细胞毒性
反式-铂(II)配合物恶二唑啉用7-硝基-1,3,5- triazaadamantane(NO 2 -TAA)或六亚甲基四胺(HMTA)配位体已经从合成反式- [氯铂酸2(PHCN)2 ]通过硝酮的环加成到一个配位的腈中,然后用NO 2 -TAA或hmta交换另一个腈。化学计量控制允许选择性合成单核和双核配合物,其中7-NO 2 TAA和hmta分别充当单和二齿配体。前体和目标配合物反式-[PtCl 2(hmta)(恶二唑啉)],反式-[PtCl 2(NO 2 -TAA)(恶二唑啉)]和反式-[{PtCl 2(恶二唑啉)} 2(hmta)]的元素分析,IR和多核(1 H,13 C,195 Pt)NMR表征光谱学。DFT(B3LYP / 6-31G * / LANL08)和AIM计算表明hmta与[PtCl 2(恶二唑啉)]片段的结合更牢固,与配体交换中实验观察到的反应性一致(hmta> 7-NO 2 TAA )。预计用hmta代替腈会比用7-NO 2放热更多。-TAA,尽管激活障碍相似。预期非配位的N原子的质子化会削弱Pt–N键并降低配体交换的激活势垒。这种作用可能有助于在弱酸性环境(例如某些肿瘤组织)中激活这些化合物。测试了十种新化合物在人癌细胞系HeLa和A549中的体外细胞毒性。一些单核复合物比顺铂更有效,并且它们的活性在A549中仍然很高,而顺铂几乎没有作用。双核复合物是无活性的,大概是由于它们的亲脂性和在水中的溶解度降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号