当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A new selective route towards benzoic acid and derivatives from biomass-derived coumalic acid
Green Chemistry ( IF 9.3 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1039/c7gc02041d Toni Pfennig 1, 2, 3, 4, 5 , Jack M. Carraher 1, 2, 3, 4, 5 , Ashwin Chemburkar 3, 4, 5, 6, 7 , Robert L. Johnson 1, 2, 3, 4, 5 , Austin T. Anderson 1, 2, 3, 4, 5 , Jean-Philippe Tessonnier 1, 2, 3, 4, 5 , Matthew Neurock 3, 4, 5, 6, 7 , Brent H. Shanks 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1039/c7gc02041d Toni Pfennig 1, 2, 3, 4, 5 , Jack M. Carraher 1, 2, 3, 4, 5 , Ashwin Chemburkar 3, 4, 5, 6, 7 , Robert L. Johnson 1, 2, 3, 4, 5 , Austin T. Anderson 1, 2, 3, 4, 5 , Jean-Philippe Tessonnier 1, 2, 3, 4, 5 , Matthew Neurock 3, 4, 5, 6, 7 , Brent H. Shanks 1, 2, 3, 4, 5
Affiliation
|
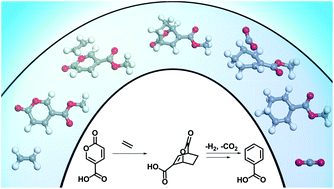
The selective production of aromatics from bio-based sources is an area of interest to expand the potential for greener alternatives to petroleum-derived chemicals. A scalable, efficient route to produce bio-based benzoates is demonstrated by carrying out heterogeneous catalytic reactions in non-toxic bio-based solvents at 180 °C obtaining yields of up to 100 mol%. This approach extends the 2-pyrone (coumalic acid/methyl coumalate) Diels–Alder platform by utilizing a bioavailable co-reactant ethylene. A detailed investigation using a combination of kinetic experiments, DFT calculations, and multi-dimensional NMR was carried out to determine the detailed reaction network, and the corresponding activation energies for critical steps. Additionally, a series of experiments were conducted to maximize the yields by comparing different solvents, for both coumalic acid and methyl coumalate. Our results show that the choice of solvent was a significant factor when coumalic acid was the reactant (yields 71–92 mol%), while methyl coumalate was only minimally affected by the solvent (yields 95–100 mol%). Interestingly, the reaction network and kinetic analysis showed that the Diels–Alder reactions were not significantly different between coumalic acid and methyl coumalate, with the rate limiting step for both being decarboxylation with an activation barrier of 141 kJ mol−1 compared to 77 kJ mol−1 for the formation of the bicyclic adduct. Finally, the reaction cascade was found to be highly susceptible to by-product formation when as little as 5 vol% water was present in the solvent, which demonstrates that the absence of water is essential for high yielding benzoate production.
中文翻译:

从生物质衍生的香豆酸向苯甲酸及其衍生物的新选择途径
从生物基来源选择性生产芳香剂是扩大石油衍生化学品的绿色替代品潜力的关注领域。通过在无毒的生物基溶剂中于180°C进行多相催化反应,获得了最高100 mol%的产率,证明了生产生物基苯甲酸酯的可扩展的有效途径。这种方法通过利用可生物利用的共反应性乙烯来扩展2-吡喃酮(香豆酸/香豆酸甲酯)Diels-Alder平台。结合动力学实验,DFT计算和多维NMR进行了详细研究,以确定详细的反应网络以及关键步骤的相应活化能。此外,还进行了一系列实验,通过比较不同的溶剂来最大程度地提高收率,用于香豆酸和香豆酸甲酯。我们的结果表明,当香豆酸为反应物(收率71–92 mol%)时,溶剂的选择是一个重要因素(而香豆酸甲酯受溶剂的影响很小(收率95–100 mol%))。有趣的是,反应网络和动力学分析表明,香豆酸和香豆酸甲酯之间的Diels-Alder反应没有显着差异,两者的限速步骤均为脱羧,活化障碍为141 kJ mol。-1相比77千焦耳摩尔-1的双环加合物的形成。最后,当溶剂中的水含量低至5%(体积)时,发现反应级联对副产物的形成高度敏感,这表明水的缺乏对于高产率的苯甲酸酯生产至关重要。
更新日期:2017-09-19
中文翻译:

从生物质衍生的香豆酸向苯甲酸及其衍生物的新选择途径
从生物基来源选择性生产芳香剂是扩大石油衍生化学品的绿色替代品潜力的关注领域。通过在无毒的生物基溶剂中于180°C进行多相催化反应,获得了最高100 mol%的产率,证明了生产生物基苯甲酸酯的可扩展的有效途径。这种方法通过利用可生物利用的共反应性乙烯来扩展2-吡喃酮(香豆酸/香豆酸甲酯)Diels-Alder平台。结合动力学实验,DFT计算和多维NMR进行了详细研究,以确定详细的反应网络以及关键步骤的相应活化能。此外,还进行了一系列实验,通过比较不同的溶剂来最大程度地提高收率,用于香豆酸和香豆酸甲酯。我们的结果表明,当香豆酸为反应物(收率71–92 mol%)时,溶剂的选择是一个重要因素(而香豆酸甲酯受溶剂的影响很小(收率95–100 mol%))。有趣的是,反应网络和动力学分析表明,香豆酸和香豆酸甲酯之间的Diels-Alder反应没有显着差异,两者的限速步骤均为脱羧,活化障碍为141 kJ mol。-1相比77千焦耳摩尔-1的双环加合物的形成。最后,当溶剂中的水含量低至5%(体积)时,发现反应级联对副产物的形成高度敏感,这表明水的缺乏对于高产率的苯甲酸酯生产至关重要。









































 京公网安备 11010802027423号
京公网安备 11010802027423号