当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A multidisciplinary investigation of the technical and environmental performances of TAML/peroxide elimination of Bisphenol A compounds from water
Green Chemistry ( IF 9.3 ) Pub Date : 2017-08-02 00:00:00 , DOI: 10.1039/c7gc01415e Yusuf Onundi 1, 2, 3, 4 , Bethany A. Drake 5, 6, 7, 8, 9 , Ryan T. Malecky 5, 6, 7, 8, 9 , Matthew A. DeNardo 5, 6, 7, 8, 9 , Matthew R. Mills 5, 6, 7, 8, 9 , Soumen Kundu 5, 6, 7, 8, 9 , Alexander D. Ryabov 5, 6, 7, 8, 9 , Evan S. Beach 5, 6, 7, 8, 9 , Colin P. Horwitz 5, 6, 7, 8, 9 , Michael T. Simonich 10, 11, 12, 13, 14 , Lisa Truong 10, 11, 12, 13, 14 , Robert L. Tanguay 10, 11, 12, 13, 14 , L. James Wright 2, 3, 4, 15, 16 , Naresh Singhal 1, 2, 3, 4 , Terrence J. Collins 5, 6, 7, 8, 9
Green Chemistry ( IF 9.3 ) Pub Date : 2017-08-02 00:00:00 , DOI: 10.1039/c7gc01415e Yusuf Onundi 1, 2, 3, 4 , Bethany A. Drake 5, 6, 7, 8, 9 , Ryan T. Malecky 5, 6, 7, 8, 9 , Matthew A. DeNardo 5, 6, 7, 8, 9 , Matthew R. Mills 5, 6, 7, 8, 9 , Soumen Kundu 5, 6, 7, 8, 9 , Alexander D. Ryabov 5, 6, 7, 8, 9 , Evan S. Beach 5, 6, 7, 8, 9 , Colin P. Horwitz 5, 6, 7, 8, 9 , Michael T. Simonich 10, 11, 12, 13, 14 , Lisa Truong 10, 11, 12, 13, 14 , Robert L. Tanguay 10, 11, 12, 13, 14 , L. James Wright 2, 3, 4, 15, 16 , Naresh Singhal 1, 2, 3, 4 , Terrence J. Collins 5, 6, 7, 8, 9
Affiliation
|
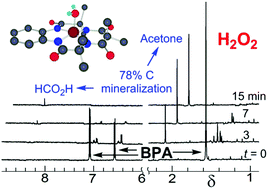
Designing technologies that mitigate the low-dose adverse effects of exposures to large-volume, everyday-everywhere chemicals such as bisphenol A (BPA, 1a) requires an understanding of the scope of the exposures and the nature of the adverse effects. Therefore, we review the literature of, (i) the occurrences of 1a in humans, waters and products and the effectiveness of widely deployed mitigation methods in 1a stewardship and, (ii) the adverse effects of 1a exposures on human cells and fish. Within this broad context, we present and evaluate experimental results on TAML/H2O2 purification of 1a contaminated waters. TAML/H2O2 catalysis readily oxidizes BPA (1a) and the ring-tetramethyl (1b), tetrachloro (1c), and tetrabromo (1d)-substituted derivatives. At pH 8.5, TAML/H2O2 induces controllable, oxidative oligomerisation of 1a (2-, 3-, 4-, and 5-unit species were identified) with precipitation, establishing a green synthetic pathway to these substances for biological safety characterisation and an easy method for near quantitative removal of 1a from water. TAML/H2O2 (24 nM/4 mM) treatment of 1a (10 000 μg L−1) in pH 8.5 (0.01 M, carbonate) lab water effects a >99% reduction (to <100 μg L−11a) within 30 min. Yeast oestrogen screens (YES) of the pH 8.5, TAML/H2O2 treated, catalase quenched, and filtered oxidation solutions show elimination of 1a oestrogenicity. Zebrafish developmental assays of TAML/H2O2 treated, unfiltered, agitated pH 7, 1a solutions showed no significant incidences of abnormality among any of 22 endpoints—treated samples showed an insignificant increase in mortality. At pH 11, the TAML/H2O2 oxidations of 1a–d are fast with second order rate constants for the substrate oxidation process (kII values) of (0.57–8) × 104 M−1 s−1. The 1a oxidation gives CO and CO2 (∼78%), acetone (∼25%) and formate (∼1%). In striking contrast with pH 8.5 treatment, no oligomers were detected. TAML/H2O2 (150 nM/7.5 mM) treatment of 1a (34
000 μg L−1) in pH 8.5 (0.01 M, carbonate) lab water effects a >99% reduction (to <100 μg L−11a) within 30 min. Yeast oestrogen screens (YES) of the pH 8.5, TAML/H2O2 treated, catalase quenched, and filtered oxidation solutions show elimination of 1a oestrogenicity. Zebrafish developmental assays of TAML/H2O2 treated, unfiltered, agitated pH 7, 1a solutions showed no significant incidences of abnormality among any of 22 endpoints—treated samples showed an insignificant increase in mortality. At pH 11, the TAML/H2O2 oxidations of 1a–d are fast with second order rate constants for the substrate oxidation process (kII values) of (0.57–8) × 104 M−1 s−1. The 1a oxidation gives CO and CO2 (∼78%), acetone (∼25%) and formate (∼1%). In striking contrast with pH 8.5 treatment, no oligomers were detected. TAML/H2O2 (150 nM/7.5 mM) treatment of 1a (34 244 μg L−1) in pH 11 (0.01 M, phosphate) lab water effected a >99.9% reduction (to <23 μg L−11a) within 15 min. The pH dependent behaviour of 1a was examined as a possible origin of the differing outcomes. The 1st and 2nd pKa values of 1a were estimated by fitting the pH dependence of the UV-vis spectra (pKa1 = 9.4 ± 0.3; pKa2 = 10.37 ± 0.07). At pH 8.5, coupling of the radical produced on initial oxidation evidently outcompetes further oxidation. A linear free energy relationship between the logarithm of the pH 11, kII values and the redox potentials of 1a–d as determined by differential pulse voltammetry in CH3CN is consistent with rate-limiting, electron transfer from the dianionic form of 1a at pH 11, followed by a multistep, deep degradation without observation of 4-(prop-1-en-2-yl)phenol 12, a common 1a oxidation product—an improved synthesis of 12 is described. Microtox® analyses of pH 12, TAML/H2O2 treated 1a solutions showed significantly reduced toxicity. The facility and high efficiency by which TAML/H2O2 catalysis eliminates 1a from water, by either mechanism, suggests a new and simple procedure for 1a stewardship.
244 μg L−1) in pH 11 (0.01 M, phosphate) lab water effected a >99.9% reduction (to <23 μg L−11a) within 15 min. The pH dependent behaviour of 1a was examined as a possible origin of the differing outcomes. The 1st and 2nd pKa values of 1a were estimated by fitting the pH dependence of the UV-vis spectra (pKa1 = 9.4 ± 0.3; pKa2 = 10.37 ± 0.07). At pH 8.5, coupling of the radical produced on initial oxidation evidently outcompetes further oxidation. A linear free energy relationship between the logarithm of the pH 11, kII values and the redox potentials of 1a–d as determined by differential pulse voltammetry in CH3CN is consistent with rate-limiting, electron transfer from the dianionic form of 1a at pH 11, followed by a multistep, deep degradation without observation of 4-(prop-1-en-2-yl)phenol 12, a common 1a oxidation product—an improved synthesis of 12 is described. Microtox® analyses of pH 12, TAML/H2O2 treated 1a solutions showed significantly reduced toxicity. The facility and high efficiency by which TAML/H2O2 catalysis eliminates 1a from water, by either mechanism, suggests a new and simple procedure for 1a stewardship.
中文翻译:

TAML /过氧化物从水中消除双酚A化合物的技术和环境性能的多学科研究
设计减轻暴露于大剂量,每天到处无处不在的化学物质(如双酚A(BPA,1a))的低剂量不利影响的技术,需要了解暴露的范围和不利影响的性质。因此,我们回顾了以下文献:(i)1a在人类,水域和产品中的发生以及在1a管理中广泛采用的缓解方法的有效性,以及(ii)1a暴露对人体细胞和鱼类的不利影响。在此广阔的背景下,我们提出并评估了1a污染水的TAML / H 2 O 2纯化的实验结果。TAML / H 2 O2催化容易氧化BPA( 1a)和环四甲基( 1b),四氯( 1c)和四溴( 1d)取代的衍生物。在pH 8.5时,TAML / H 2 O 2诱导1a(可识别出2-,3-,4-和5-单元物种)的可控氧化寡聚,从而为这些物质建立绿色合成途径,以进行生物学安全性鉴定一种简便的从水中几乎定量去除1a的方法。TAML / H 2 ö 2(24纳米/ 4毫米)治疗的1A(10 000微克大号-1)在pH 8.5(0.01 M,碳酸盐)的实验室水中在30分钟内降低了> 99%(降至<100μgL -1 1a)。pH 8.5,经TAML / H 2 O 2处理,过氧化氢酶淬灭和过滤的氧化溶液的酵母雌激素筛选(YES)显示消除了1a雌激素性。TAML的斑马鱼发育测定/ H 2 ö 2处理,未过滤的,搅拌pH为7,1a中的解决方案显示出异常的无显著发生率中的任意的22端点处理的样品显示出显着的升高的死亡率。在pH 11时,TAML / H 2 O 2氧化为1a–d衬底氧化过程的二阶速率常数(k II值)为(0.57–8)×10 4 M -1 s -1很快。在1A氧化得到的CO和CO 2(~78%),丙酮(〜25%)和甲酸(〜1%)。与pH 8.5处理形成鲜明对比的是,未检测到任何低聚物。TAML / H 2 ö 2(150纳米/ 7.5毫米)治疗的1A(34
000微克大号-1)在pH 8.5(0.01 M,碳酸盐)的实验室水中在30分钟内降低了> 99%(降至<100μgL -1 1a)。pH 8.5,经TAML / H 2 O 2处理,过氧化氢酶淬灭和过滤的氧化溶液的酵母雌激素筛选(YES)显示消除了1a雌激素性。TAML的斑马鱼发育测定/ H 2 ö 2处理,未过滤的,搅拌pH为7,1a中的解决方案显示出异常的无显著发生率中的任意的22端点处理的样品显示出显着的升高的死亡率。在pH 11时,TAML / H 2 O 2氧化为1a–d衬底氧化过程的二阶速率常数(k II值)为(0.57–8)×10 4 M -1 s -1很快。在1A氧化得到的CO和CO 2(~78%),丙酮(〜25%)和甲酸(〜1%)。与pH 8.5处理形成鲜明对比的是,未检测到任何低聚物。TAML / H 2 ö 2(150纳米/ 7.5毫米)治疗的1A(34  244微克大号-1)在pH值为11(0.01M,磷酸盐)实验室水进行一> 99.9%的减少(〜<23微克大号-1 1A)在15分钟内。1a的pH依赖行为被检查为不同结果可能的起源。1日和2次p ķ一个的值1A通过拟合的UV-vis光谱(p的pH依赖性估计ķ A1 ; P = 9.4±0.3 ķ A2 = 10.37±0.07)。在pH 8.5时,初始氧化产生的自由基的偶联明显超过了进一步的氧化。由CH 3 CN中的差动脉冲伏安法测定的pH 11的对数和k II值与1a–d的氧化还原电势之间的线性自由能关系与限速,电子从二价阴离子形式的转移有关1a在pH值为11的情况下,随后进行了多步深度降解,而没有观察到4-(prop-1-en-2-yl)phenol 12(一种常见的1a氧化产物)—改进了12的合成。pH 12,经TAML / H 2 O 2处理的1a溶液的Microtox®分析显示毒性显着降低。TAML / H 2 O 2催化通过任何一种机理从水中消除1a的便利性和高效率,为1a的管理提出了一种新的简单程序。
244微克大号-1)在pH值为11(0.01M,磷酸盐)实验室水进行一> 99.9%的减少(〜<23微克大号-1 1A)在15分钟内。1a的pH依赖行为被检查为不同结果可能的起源。1日和2次p ķ一个的值1A通过拟合的UV-vis光谱(p的pH依赖性估计ķ A1 ; P = 9.4±0.3 ķ A2 = 10.37±0.07)。在pH 8.5时,初始氧化产生的自由基的偶联明显超过了进一步的氧化。由CH 3 CN中的差动脉冲伏安法测定的pH 11的对数和k II值与1a–d的氧化还原电势之间的线性自由能关系与限速,电子从二价阴离子形式的转移有关1a在pH值为11的情况下,随后进行了多步深度降解,而没有观察到4-(prop-1-en-2-yl)phenol 12(一种常见的1a氧化产物)—改进了12的合成。pH 12,经TAML / H 2 O 2处理的1a溶液的Microtox®分析显示毒性显着降低。TAML / H 2 O 2催化通过任何一种机理从水中消除1a的便利性和高效率,为1a的管理提出了一种新的简单程序。
更新日期:2017-09-19
 000 μg L−1) in pH 8.5 (0.01 M, carbonate) lab water effects a >99% reduction (to <100 μg L−11a) within 30 min. Yeast oestrogen screens (YES) of the pH 8.5, TAML/H2O2 treated, catalase quenched, and filtered oxidation solutions show elimination of 1a oestrogenicity. Zebrafish developmental assays of TAML/H2O2 treated, unfiltered, agitated pH 7, 1a solutions showed no significant incidences of abnormality among any of 22 endpoints—treated samples showed an insignificant increase in mortality. At pH 11, the TAML/H2O2 oxidations of 1a–d are fast with second order rate constants for the substrate oxidation process (kII values) of (0.57–8) × 104 M−1 s−1. The 1a oxidation gives CO and CO2 (∼78%), acetone (∼25%) and formate (∼1%). In striking contrast with pH 8.5 treatment, no oligomers were detected. TAML/H2O2 (150 nM/7.5 mM) treatment of 1a (34
000 μg L−1) in pH 8.5 (0.01 M, carbonate) lab water effects a >99% reduction (to <100 μg L−11a) within 30 min. Yeast oestrogen screens (YES) of the pH 8.5, TAML/H2O2 treated, catalase quenched, and filtered oxidation solutions show elimination of 1a oestrogenicity. Zebrafish developmental assays of TAML/H2O2 treated, unfiltered, agitated pH 7, 1a solutions showed no significant incidences of abnormality among any of 22 endpoints—treated samples showed an insignificant increase in mortality. At pH 11, the TAML/H2O2 oxidations of 1a–d are fast with second order rate constants for the substrate oxidation process (kII values) of (0.57–8) × 104 M−1 s−1. The 1a oxidation gives CO and CO2 (∼78%), acetone (∼25%) and formate (∼1%). In striking contrast with pH 8.5 treatment, no oligomers were detected. TAML/H2O2 (150 nM/7.5 mM) treatment of 1a (34 244 μg L−1) in pH 11 (0.01 M, phosphate) lab water effected a >99.9% reduction (to <23 μg L−11a) within 15 min. The pH dependent behaviour of 1a was examined as a possible origin of the differing outcomes. The 1st and 2nd pKa values of 1a were estimated by fitting the pH dependence of the UV-vis spectra (pKa1 = 9.4 ± 0.3; pKa2 = 10.37 ± 0.07). At pH 8.5, coupling of the radical produced on initial oxidation evidently outcompetes further oxidation. A linear free energy relationship between the logarithm of the pH 11, kII values and the redox potentials of 1a–d as determined by differential pulse voltammetry in CH3CN is consistent with rate-limiting, electron transfer from the dianionic form of 1a at pH 11, followed by a multistep, deep degradation without observation of 4-(prop-1-en-2-yl)phenol 12, a common 1a oxidation product—an improved synthesis of 12 is described. Microtox® analyses of pH 12, TAML/H2O2 treated 1a solutions showed significantly reduced toxicity. The facility and high efficiency by which TAML/H2O2 catalysis eliminates 1a from water, by either mechanism, suggests a new and simple procedure for 1a stewardship.
244 μg L−1) in pH 11 (0.01 M, phosphate) lab water effected a >99.9% reduction (to <23 μg L−11a) within 15 min. The pH dependent behaviour of 1a was examined as a possible origin of the differing outcomes. The 1st and 2nd pKa values of 1a were estimated by fitting the pH dependence of the UV-vis spectra (pKa1 = 9.4 ± 0.3; pKa2 = 10.37 ± 0.07). At pH 8.5, coupling of the radical produced on initial oxidation evidently outcompetes further oxidation. A linear free energy relationship between the logarithm of the pH 11, kII values and the redox potentials of 1a–d as determined by differential pulse voltammetry in CH3CN is consistent with rate-limiting, electron transfer from the dianionic form of 1a at pH 11, followed by a multistep, deep degradation without observation of 4-(prop-1-en-2-yl)phenol 12, a common 1a oxidation product—an improved synthesis of 12 is described. Microtox® analyses of pH 12, TAML/H2O2 treated 1a solutions showed significantly reduced toxicity. The facility and high efficiency by which TAML/H2O2 catalysis eliminates 1a from water, by either mechanism, suggests a new and simple procedure for 1a stewardship.
中文翻译:

TAML /过氧化物从水中消除双酚A化合物的技术和环境性能的多学科研究
设计减轻暴露于大剂量,每天到处无处不在的化学物质(如双酚A(BPA,1a))的低剂量不利影响的技术,需要了解暴露的范围和不利影响的性质。因此,我们回顾了以下文献:(i)1a在人类,水域和产品中的发生以及在1a管理中广泛采用的缓解方法的有效性,以及(ii)1a暴露对人体细胞和鱼类的不利影响。在此广阔的背景下,我们提出并评估了1a污染水的TAML / H 2 O 2纯化的实验结果。TAML / H 2 O2催化容易氧化BPA( 1a)和环四甲基( 1b),四氯( 1c)和四溴( 1d)取代的衍生物。在pH 8.5时,TAML / H 2 O 2诱导1a(可识别出2-,3-,4-和5-单元物种)的可控氧化寡聚,从而为这些物质建立绿色合成途径,以进行生物学安全性鉴定一种简便的从水中几乎定量去除1a的方法。TAML / H 2 ö 2(24纳米/ 4毫米)治疗的1A(10
 000微克大号-1)在pH 8.5(0.01 M,碳酸盐)的实验室水中在30分钟内降低了> 99%(降至<100μgL -1 1a)。pH 8.5,经TAML / H 2 O 2处理,过氧化氢酶淬灭和过滤的氧化溶液的酵母雌激素筛选(YES)显示消除了1a雌激素性。TAML的斑马鱼发育测定/ H 2 ö 2处理,未过滤的,搅拌pH为7,1a中的解决方案显示出异常的无显著发生率中的任意的22端点处理的样品显示出显着的升高的死亡率。在pH 11时,TAML / H 2 O 2氧化为1a–d衬底氧化过程的二阶速率常数(k II值)为(0.57–8)×10 4 M -1 s -1很快。在1A氧化得到的CO和CO 2(~78%),丙酮(〜25%)和甲酸(〜1%)。与pH 8.5处理形成鲜明对比的是,未检测到任何低聚物。TAML / H 2 ö 2(150纳米/ 7.5毫米)治疗的1A(34
000微克大号-1)在pH 8.5(0.01 M,碳酸盐)的实验室水中在30分钟内降低了> 99%(降至<100μgL -1 1a)。pH 8.5,经TAML / H 2 O 2处理,过氧化氢酶淬灭和过滤的氧化溶液的酵母雌激素筛选(YES)显示消除了1a雌激素性。TAML的斑马鱼发育测定/ H 2 ö 2处理,未过滤的,搅拌pH为7,1a中的解决方案显示出异常的无显著发生率中的任意的22端点处理的样品显示出显着的升高的死亡率。在pH 11时,TAML / H 2 O 2氧化为1a–d衬底氧化过程的二阶速率常数(k II值)为(0.57–8)×10 4 M -1 s -1很快。在1A氧化得到的CO和CO 2(~78%),丙酮(〜25%)和甲酸(〜1%)。与pH 8.5处理形成鲜明对比的是,未检测到任何低聚物。TAML / H 2 ö 2(150纳米/ 7.5毫米)治疗的1A(34  244微克大号-1)在pH值为11(0.01M,磷酸盐)实验室水进行一> 99.9%的减少(〜<23微克大号-1 1A)在15分钟内。1a的pH依赖行为被检查为不同结果可能的起源。1日和2次p ķ一个的值1A通过拟合的UV-vis光谱(p的pH依赖性估计ķ A1 ; P = 9.4±0.3 ķ A2 = 10.37±0.07)。在pH 8.5时,初始氧化产生的自由基的偶联明显超过了进一步的氧化。由CH 3 CN中的差动脉冲伏安法测定的pH 11的对数和k II值与1a–d的氧化还原电势之间的线性自由能关系与限速,电子从二价阴离子形式的转移有关1a在pH值为11的情况下,随后进行了多步深度降解,而没有观察到4-(prop-1-en-2-yl)phenol 12(一种常见的1a氧化产物)—改进了12的合成。pH 12,经TAML / H 2 O 2处理的1a溶液的Microtox®分析显示毒性显着降低。TAML / H 2 O 2催化通过任何一种机理从水中消除1a的便利性和高效率,为1a的管理提出了一种新的简单程序。
244微克大号-1)在pH值为11(0.01M,磷酸盐)实验室水进行一> 99.9%的减少(〜<23微克大号-1 1A)在15分钟内。1a的pH依赖行为被检查为不同结果可能的起源。1日和2次p ķ一个的值1A通过拟合的UV-vis光谱(p的pH依赖性估计ķ A1 ; P = 9.4±0.3 ķ A2 = 10.37±0.07)。在pH 8.5时,初始氧化产生的自由基的偶联明显超过了进一步的氧化。由CH 3 CN中的差动脉冲伏安法测定的pH 11的对数和k II值与1a–d的氧化还原电势之间的线性自由能关系与限速,电子从二价阴离子形式的转移有关1a在pH值为11的情况下,随后进行了多步深度降解,而没有观察到4-(prop-1-en-2-yl)phenol 12(一种常见的1a氧化产物)—改进了12的合成。pH 12,经TAML / H 2 O 2处理的1a溶液的Microtox®分析显示毒性显着降低。TAML / H 2 O 2催化通过任何一种机理从水中消除1a的便利性和高效率,为1a的管理提出了一种新的简单程序。











































 京公网安备 11010802027423号
京公网安备 11010802027423号