当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective conversion of bio-derived ethanol to renewable BTX over Ga-ZSM-5
Green Chemistry ( IF 9.3 ) Pub Date : 2017-06-01 00:00:00 , DOI: 10.1039/c7gc01188a Zhenglong Li 1, 2, 3, 4 , Andrew W. Lepore 2, 3, 4, 5, 6 , Mariam F. Salazar 2, 3, 4, 5 , Guo Shiou Foo 2, 3, 4, 7 , Brian H. Davison 2, 3, 4, 8 , Zili Wu 2, 3, 4, 7, 9 , Chaitanya K. Narula 2, 3, 4, 5, 6
Green Chemistry ( IF 9.3 ) Pub Date : 2017-06-01 00:00:00 , DOI: 10.1039/c7gc01188a Zhenglong Li 1, 2, 3, 4 , Andrew W. Lepore 2, 3, 4, 5, 6 , Mariam F. Salazar 2, 3, 4, 5 , Guo Shiou Foo 2, 3, 4, 7 , Brian H. Davison 2, 3, 4, 8 , Zili Wu 2, 3, 4, 7, 9 , Chaitanya K. Narula 2, 3, 4, 5, 6
Affiliation
|
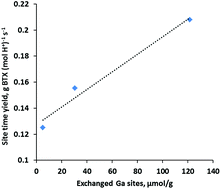
Selective conversion of bio-derived ethanol to benzene, toluene and xylenes (BTX) is desirable for producing renewable BTX. In this work, we show that addition of Ga to H-ZSM-5 leads to a two-fold increase in the BTX yield as compared with H-ZSM-5 when ethanol is converted over these zeolites at 450 °C and ambient pressure. Besides promoting BTX formation, Ga also plays an important role in enhancing molecular hydrogen production and suppressing hydrogen transfer reactions for light alkane formation. The ion exchange synthesis of Ga-ZSM-5 results in the majority of Ga at the outer surface of zeolite crystals as extra-zeolitic Ga2O3 particles and only a small fraction of Ga exchanging with the Brønsted acid sites which appears to be responsible for higher ethanol conversion to BTX. The interface between H-ZSM-5 and Ga2O3 particles is not active since H-ZSM-5 and the physical mixture of β-Ga2O3/H-ZSM-5 furnish an almost identical product distribution. Hydrogen reduction of the physical mixtures facilitates movement of Ga to ion exchange locations and dramatically increases the BTX yield becoming comparable to those obtained over ion-exchanged Ga-ZSM-5, suggesting that exchanged Ga(III) cations are responsible for the increased BTX production. A linear correlation between the BTX site time yield and exchanged Ga sites further confirms that Ga occupying cationic sites are active sites for enhancing BTX formation. Reduction of physical mixtures (β-Ga2O3/H-ZSM-5) also provides an economical and environmentally friendly non-aqueous method for large scale catalyst synthesis without sacrificing catalyst performance for ethanol conversion application.
中文翻译:

通过Ga-ZSM-5将生物来源的乙醇选择性转化为可再生的BTX
生物来源的乙醇选择性转化为苯,甲苯和二甲苯(BTX)对于生产可再生的BTX是理想的。在这项工作中,我们表明,当乙醇在450°C和环境压力下通过这些沸石转化时,与H-ZSM-5相比,向H-ZSM-5中添加Ga会导致BTX收率提高两倍。除了促进BTX的形成外,Ga在提高分子氢的产生和抑制氢转移反应以形成轻质烷烃方面也起着重要的作用。Ga-ZSM-5的离子交换合成导致大部分Ga在沸石晶体的外表面以沸石外Ga 2 O 3的形式存在。颗粒和仅一小部分的Ga与布朗斯台德酸位交换,这似乎是导致乙醇向BTX转化的程度更高的原因。H-ZSM-5和Ga之间的界面2个ö 3颗粒是不活动,因为H-ZSM-5和β-Ga构成的物理混合物2 ö 3 / H-ZSM-5配料几乎相同的产物分布。物理混合物的氢还原作用有利于Ga移至离子交换位置,并显着提高BTX产量,使其与通过离子交换的Ga-ZSM-5所获得的产率相当,这表明被交换的Ga(III)阳离子是导致BTX产量增加的原因。BTX位点时间产量与交换的Ga位点之间的线性相关性进一步证实,Ga占据的阳离子位点是增强BTX形成的活性位点。物理混合物的还原(的β-Ga 2 ö 3 / H-ZSM-5)还提供了用于大规模合成催化剂而不牺牲催化剂性能乙醇转化应用的经济和环保的非水性方法。
更新日期:2017-09-19
中文翻译:

通过Ga-ZSM-5将生物来源的乙醇选择性转化为可再生的BTX
生物来源的乙醇选择性转化为苯,甲苯和二甲苯(BTX)对于生产可再生的BTX是理想的。在这项工作中,我们表明,当乙醇在450°C和环境压力下通过这些沸石转化时,与H-ZSM-5相比,向H-ZSM-5中添加Ga会导致BTX收率提高两倍。除了促进BTX的形成外,Ga在提高分子氢的产生和抑制氢转移反应以形成轻质烷烃方面也起着重要的作用。Ga-ZSM-5的离子交换合成导致大部分Ga在沸石晶体的外表面以沸石外Ga 2 O 3的形式存在。颗粒和仅一小部分的Ga与布朗斯台德酸位交换,这似乎是导致乙醇向BTX转化的程度更高的原因。H-ZSM-5和Ga之间的界面2个ö 3颗粒是不活动,因为H-ZSM-5和β-Ga构成的物理混合物2 ö 3 / H-ZSM-5配料几乎相同的产物分布。物理混合物的氢还原作用有利于Ga移至离子交换位置,并显着提高BTX产量,使其与通过离子交换的Ga-ZSM-5所获得的产率相当,这表明被交换的Ga(III)阳离子是导致BTX产量增加的原因。BTX位点时间产量与交换的Ga位点之间的线性相关性进一步证实,Ga占据的阳离子位点是增强BTX形成的活性位点。物理混合物的还原(的β-Ga 2 ö 3 / H-ZSM-5)还提供了用于大规模合成催化剂而不牺牲催化剂性能乙醇转化应用的经济和环保的非水性方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号