当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Reactant and Product State Decay on Ultrafast Charge-Transfer Kinetics: Violation of the Principle of Independence of Elementary Chemical Reactions
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.jpcc.7b06106 Valentina A. Mikhailova 1 , Anatoly I. Ivanov 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.jpcc.7b06106 Valentina A. Mikhailova 1 , Anatoly I. Ivanov 1
Affiliation
|
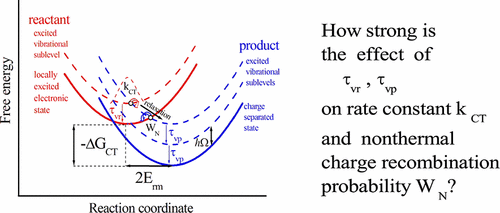
Fast decay of both the reactant and product states is shown to strongly increase the intrinsic electron transfer rate in donor–acceptor dyads. The decay is associated with redistribution/relaxation of excited vibrational states that typically participate in the reaction. Although the role of the reorganization of high-frequency vibrational modes in electron-transfer dynamics is well understood and it is commonly accepted to strongly affect the ultrafast electron transfer dynamics, the influence of the relaxation of excited vibrational states on electron transfer is not accounted for in experimental data analysis. In photoinduced electron transfer, excited states of high-frequency vibrational modes are often produced by laser pump pulse so that the ultrafast charge separation, at least partly, occurs from excited vibrational states. The charge recombination accompanying the charge separation also essentially occurs from excited vibrational states of intermediates to form a final excited vibrational state. Since the decay of the reactant state and electron transfer are two elementary chemical reactions occurring in parallel, the influence of vibrational relaxation on the intrinsic electron-transfer rate constant is a clear manifestation of the violation of the fundamental principle of chemical kinetics postulating the independence of elementary chemical reactions. The mechanism of the violation is discussed in detail. The transition probability that better characterizes the efficiency of ultrafast nonequilibrium charge recombination than the rate constant is calculated. The dependencies of the electron-transfer rate constant and the transition probability on the product decay time are predicted to be identical while on the reactant decay time to be opposite.
中文翻译:

反应物和产物状态衰减对超快电荷转移动力学的影响:违反基本化学反应独立性原理
结果表明,反应物和产物状态的快速衰减都可以大大提高供体-受体二元组中的固有电子传递速率。衰减与通常参与反应的激发振动态的重新分布/松弛相关。尽管人们已经很好地理解了高频振动模式的重组在电子传递动力学中的作用,并且人们普遍认为它会强烈影响超快电子传递动力学,但是并没有考虑到激发振动态的弛豫对电子传递的影响。在实验数据分析中。在光致电子转移中,高频振动模式的激发态通常是由激光泵浦脉冲产生的,因此超快电荷分离至少部分是由激发振动态引起的。伴随电荷分离的电荷复合基本上也从中间体的激发振动状态发生,以形成最终的激发振动状态。由于反应物态的衰减和电子传递是并行发生的两个基本化学反应,因此振动弛豫对本征电子传递速率常数的影响清楚地表明了违反化学动力学基本原理的假设,即假设化学反应的独立性。基本化学反应。详细讨论了违规的机制。计算出比速率常数更好地表征超快非平衡电荷重组效率的跃迁概率。
更新日期:2017-09-19
中文翻译:

反应物和产物状态衰减对超快电荷转移动力学的影响:违反基本化学反应独立性原理
结果表明,反应物和产物状态的快速衰减都可以大大提高供体-受体二元组中的固有电子传递速率。衰减与通常参与反应的激发振动态的重新分布/松弛相关。尽管人们已经很好地理解了高频振动模式的重组在电子传递动力学中的作用,并且人们普遍认为它会强烈影响超快电子传递动力学,但是并没有考虑到激发振动态的弛豫对电子传递的影响。在实验数据分析中。在光致电子转移中,高频振动模式的激发态通常是由激光泵浦脉冲产生的,因此超快电荷分离至少部分是由激发振动态引起的。伴随电荷分离的电荷复合基本上也从中间体的激发振动状态发生,以形成最终的激发振动状态。由于反应物态的衰减和电子传递是并行发生的两个基本化学反应,因此振动弛豫对本征电子传递速率常数的影响清楚地表明了违反化学动力学基本原理的假设,即假设化学反应的独立性。基本化学反应。详细讨论了违规的机制。计算出比速率常数更好地表征超快非平衡电荷重组效率的跃迁概率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号