当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid Microjet Measurements of the Entry of Organic Acids and Bases into Salty Water
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.jpcc.7b07887 Thomas B. Sobyra 1 , Matthew P. Melvin 1 , Gilbert M. Nathanson 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.jpcc.7b07887 Thomas B. Sobyra 1 , Matthew P. Melvin 1 , Gilbert M. Nathanson 1
Affiliation
|
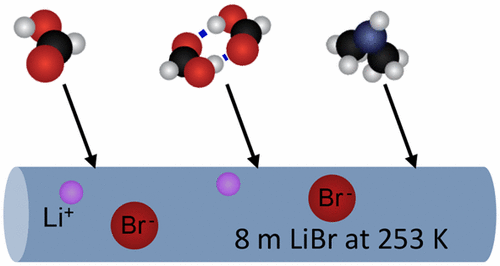
We explore collisions of hydrogen-bonding molecules with salty water using gas–microjet scattering experiments. Two aqueous solutions, 8 molal (m) LiBr/H2O and ∼4 m K2SO3/H2O at 253 K were exposed to seven organic gases representing different functional groups. These gases comprise weak acids (formic and acetic), weak bases (dimethylamine and piperidine), and an alcohol, ether, and ester (ethanol, dimethyl ether, and methyl formate). The scattering experiments are used to monitor the disappearance of each gas into the aqueous solutions over a ∼100 μs observation time. They demonstrate that formic acid and piperidine disappear into both solutions on almost every collision. Dimers of formic and acetic acid are also captured by the solutions on every collision, despite their pre-existing double hydrogen bonds. The methylene ring of piperidine, (CH2)5NH, also does not interfere with uptake. At the opposite extreme, methyl formate and dimethyl ether are so weakly soluble that they evaporate completely within the observation window, precluding the measurement of their entry probability. Ethanol and dimethylamine represent intermediate cases in which dimethylamine interacts more strongly with dissolved Li+ ions than K+ ions. Collectively, the experiments imply that organic acids and bases reach hydrogen-bonding configurations following nearly every collision, enabling them to be captured by surface water molecules.
中文翻译:

液体微喷法测量有机酸和碱进入咸水的能力
我们使用气体-微射流散射实验探索了氢键分子与盐水的碰撞。两种水溶液,8摩尔(m)LiBr / H 2 O和〜4 m K 2 SO 3 / H 2O在253 K下暴露于代表不同官能团的七种有机气体中。这些气体包括弱酸(甲酸和乙酸),弱碱(二甲胺和哌啶)以及醇,醚和酯(乙醇,二甲醚和甲酸甲酯)。散射实验用于在约100μs的观察时间内监测每种气体消失在水溶液中的情况。他们证明甲酸和哌啶几乎在每次碰撞时都消失在两种溶液中。甲酸和乙酸的二聚体在每次碰撞时也会被溶液捕获,尽管它们预先存在双氢键。哌啶亚甲基环,(CH 2)5NH,也不会干扰摄取。相反,甲酸甲酯和二甲醚的溶解度很弱,以至于它们在观察窗内完全蒸发,从而无法测量其进入概率。乙醇和二甲胺代表了中间情况,其中二甲胺与溶解的Li +离子的相互作用比K +离子更强烈。总的来说,实验暗示有机酸和碱几乎在每次碰撞后都会达到氢键构型,从而使它们能够被地表水分子捕获。
更新日期:2017-09-19
中文翻译:

液体微喷法测量有机酸和碱进入咸水的能力
我们使用气体-微射流散射实验探索了氢键分子与盐水的碰撞。两种水溶液,8摩尔(m)LiBr / H 2 O和〜4 m K 2 SO 3 / H 2O在253 K下暴露于代表不同官能团的七种有机气体中。这些气体包括弱酸(甲酸和乙酸),弱碱(二甲胺和哌啶)以及醇,醚和酯(乙醇,二甲醚和甲酸甲酯)。散射实验用于在约100μs的观察时间内监测每种气体消失在水溶液中的情况。他们证明甲酸和哌啶几乎在每次碰撞时都消失在两种溶液中。甲酸和乙酸的二聚体在每次碰撞时也会被溶液捕获,尽管它们预先存在双氢键。哌啶亚甲基环,(CH 2)5NH,也不会干扰摄取。相反,甲酸甲酯和二甲醚的溶解度很弱,以至于它们在观察窗内完全蒸发,从而无法测量其进入概率。乙醇和二甲胺代表了中间情况,其中二甲胺与溶解的Li +离子的相互作用比K +离子更强烈。总的来说,实验暗示有机酸和碱几乎在每次碰撞后都会达到氢键构型,从而使它们能够被地表水分子捕获。











































 京公网安备 11010802027423号
京公网安备 11010802027423号