当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fluorine Pseudocontact Shifts Used for Characterizing the Protein–Ligand Interaction Mode in the Limit of NMR Intermediate Exchange
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-09-19 00:35:52 , DOI: 10.1002/anie.201707114 Jia Gao 1, 2 , E Liang 3 , Rongsheng Ma 1 , Fudong Li 1 , Yixiang Liu 4 , Jiuyang Liu 1 , Ling Jiang 4 , Conggang Li 4 , Haiming Dai 2 , Jihui Wu 1 , Xuncheng Su 5 , Wei He 3 , Ke Ruan 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-09-19 00:35:52 , DOI: 10.1002/anie.201707114 Jia Gao 1, 2 , E Liang 3 , Rongsheng Ma 1 , Fudong Li 1 , Yixiang Liu 4 , Jiuyang Liu 1 , Ling Jiang 4 , Conggang Li 4 , Haiming Dai 2 , Jihui Wu 1 , Xuncheng Su 5 , Wei He 3 , Ke Ruan 1
Affiliation
|
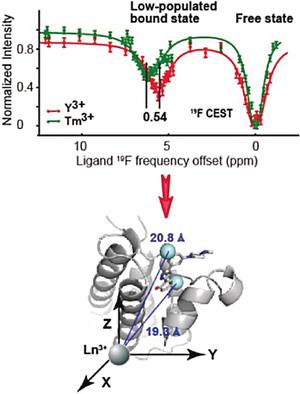
The severe line broadening in the intermediate exchange limits the applicability of NMR spectroscopy for interrogating the interaction modes of lead-like inhibitors with moderate affinities to target proteins. A 19F chemical exchange saturation transfer approach is used to retrieve the low-populated bound-state 19F pseudocontact shifts, which enable the identification of the best binding pose of the BRM bromodomain inhibitor.
中文翻译:

氟伪接触位移用于表征蛋白质-配体相互作用模式在NMR中间交换的极限。
中间交换中的严重谱线加宽限制了NMR光谱学在询问对目标蛋白具有中等亲和力的铅样抑制剂的相互作用模式时的适用性。甲19 ˚F化学交换饱和转移的方法用于检索所述低填充束缚态19 ˚Fpseudocontact移位,其使得BRM溴结构域抑制剂的最佳结合姿势的识别。
更新日期:2017-09-19
中文翻译:

氟伪接触位移用于表征蛋白质-配体相互作用模式在NMR中间交换的极限。
中间交换中的严重谱线加宽限制了NMR光谱学在询问对目标蛋白具有中等亲和力的铅样抑制剂的相互作用模式时的适用性。甲19 ˚F化学交换饱和转移的方法用于检索所述低填充束缚态19 ˚Fpseudocontact移位,其使得BRM溴结构域抑制剂的最佳结合姿势的识别。











































 京公网安备 11010802027423号
京公网安备 11010802027423号