当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing Competitive and Co-operative Hydroxyl and Ammonium Hydrogen-Bonding Directed Epoxidations
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.joc.7b01774 Marta Brambilla 1 , Méabh B. Brennan 1 , Kristína Csatayová 1 , Stephen G. Davies 1 , Ai M. Fletcher 1 , Alice M. R. Kennett 1 , James A. Lee 1 , Paul M. Roberts 1 , Angela J. Russell 1 , James E. Thomson 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.joc.7b01774 Marta Brambilla 1 , Méabh B. Brennan 1 , Kristína Csatayová 1 , Stephen G. Davies 1 , Ai M. Fletcher 1 , Alice M. R. Kennett 1 , James A. Lee 1 , Paul M. Roberts 1 , Angela J. Russell 1 , James E. Thomson 1
Affiliation
|
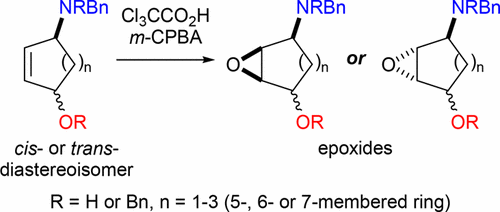
The diastereoselectivities and rates of epoxidation (upon treatment with Cl3CCO2H then m-CPBA) of a range of cis- and trans-4-aminocycloalk-2-en-1-ol derivatives (containing five-, six-, and seven-membered rings) have been investigated. In all cases where the two potential directing groups can promote epoxidation on opposite faces of the ring scaffold, evidence of competitive epoxidation pathways, promoted by hydrogen-bonding to either the in situ formed ammonium moiety or the hydroxyl group, was observed. In contrast to the relative directing group abilities already established for the six-membered ring system (NHBn ≫ OH > NBn2), an N,N-dibenzylammonium moiety appeared more proficient than a hydroxyl group at directing the stereochemical course of the epoxidation reaction in a five- or seven-membered system. In the former case, this was rationalized by the drive to minimize torsional strain in the transition state being coupled with assistance from hydrogen-bonding to the ammonium moiety. In the latter case, this was ascribed to the steric bulk of the ammonium moiety disfavoring conformations in which hydrogen-bonding to the hydroxyl group results in direction of the epoxidation to the syn face. In cases where the two potential directing groups can promote epoxidation on the same face of the ring scaffold, an enhancement of epoxidation diastereoselectivity was not observed, while introduction of a second, allylic heteroatom to the substrate results in diminishment of the rate of epoxidation in all cases. Presumably, reduction of the nucleophilicity of the olefin by the second, inductively electron-withdrawing heteroatom is the dominant factor, and any assistance to the epoxidation reaction by the potential to form hydrogen-bonds to two directing groups rather than one is clearly unable to overwhelm it.
中文翻译:

探索竞争性和合作性的氢键和氢键键合环氧化
一系列顺式和反式-4-氨基环烷-2-烯-1-醇衍生物(含五,六和六胺)的非对映选择性和环氧化速率(用Cl 3 CCO 2 H然后m -CPBA处理)七元环)已被研究。在所有两个潜在的导向基团可以促进环骨架的相反面上的环氧化的所有情况下,观察到竞争性环氧化途径的证据,该竞争性环氧化途径通过氢键结合至原位形成的铵部分或羟基而得以促进。与已经为六元环系统建立的相对指导组能力相反(NHBn = OH> NBn 2),N,N在指导五元或七元体系中环氧化反应的立体化学过程时,-二苄基铵部分似乎比羟基更熟练。在前一种情况下,通过在过渡状态下将扭转应变最小化的驱动以及与氢键合至铵部分的辅助作用相结合,可以使其合理化。在后一种情况下,这归因于铵部分的空间体积不利的构象,其中氢键合至羟基导致环氧化为顺式脸。在两个潜在的导向基团可以在环支架的同一面上促进环氧化的情况下,未观察到环氧化非对映选择性的增强,而在基质中引入第二个烯丙基杂原子会导致所有环氧化速率降低。案件。据推测,第二个感应吸电子的杂原子降低烯烃的亲核性是主要因素,并且任何可能通过与两个导向基团而不是一个与两个导向基团形成氢键的势能来辅助环氧化反应显然都无法压倒它。
更新日期:2017-09-19
中文翻译:

探索竞争性和合作性的氢键和氢键键合环氧化
一系列顺式和反式-4-氨基环烷-2-烯-1-醇衍生物(含五,六和六胺)的非对映选择性和环氧化速率(用Cl 3 CCO 2 H然后m -CPBA处理)七元环)已被研究。在所有两个潜在的导向基团可以促进环骨架的相反面上的环氧化的所有情况下,观察到竞争性环氧化途径的证据,该竞争性环氧化途径通过氢键结合至原位形成的铵部分或羟基而得以促进。与已经为六元环系统建立的相对指导组能力相反(NHBn = OH> NBn 2),N,N在指导五元或七元体系中环氧化反应的立体化学过程时,-二苄基铵部分似乎比羟基更熟练。在前一种情况下,通过在过渡状态下将扭转应变最小化的驱动以及与氢键合至铵部分的辅助作用相结合,可以使其合理化。在后一种情况下,这归因于铵部分的空间体积不利的构象,其中氢键合至羟基导致环氧化为顺式脸。在两个潜在的导向基团可以在环支架的同一面上促进环氧化的情况下,未观察到环氧化非对映选择性的增强,而在基质中引入第二个烯丙基杂原子会导致所有环氧化速率降低。案件。据推测,第二个感应吸电子的杂原子降低烯烃的亲核性是主要因素,并且任何可能通过与两个导向基团而不是一个与两个导向基团形成氢键的势能来辅助环氧化反应显然都无法压倒它。











































 京公网安备 11010802027423号
京公网安备 11010802027423号