当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Porous Metal–Organic Polyhedral Frameworks with Optimal Molecular Dynamics and Pore Geometry for Methane Storage
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/jacs.7b05453 Yong Yan 1 , Daniil I. Kolokolov 2, 3 , Ivan da Silva 4 , Alexander G. Stepanov 2, 3 , Alexander J. Blake 5 , Anne Dailly 6 , Pascal Manuel 4 , Chiu C. Tang 7 , Sihai Yang 1 , Martin Schröder 1, 8
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/jacs.7b05453 Yong Yan 1 , Daniil I. Kolokolov 2, 3 , Ivan da Silva 4 , Alexander G. Stepanov 2, 3 , Alexander J. Blake 5 , Anne Dailly 6 , Pascal Manuel 4 , Chiu C. Tang 7 , Sihai Yang 1 , Martin Schröder 1, 8
Affiliation
|
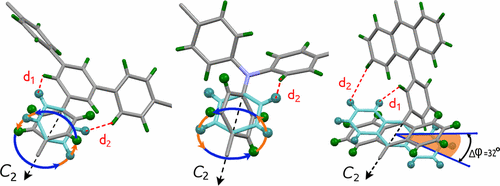
Natural gas (methane, CH4) is widely considered as a promising energy carrier for mobile applications. Maximizing the storage capacity is the primary goal for the design of future storage media. Here we report the CH4 storage properties in a family of isostructural (3,24)-connected porous materials, MFM-112a, MFM-115a, and MFM-132a, with different linker backbone functionalization. Both MFM-112a and MFM-115a show excellent CH4 uptakes of 236 and 256 cm3 (STP) cm–3 (v/v) at 80 bar and room temperature, respectively. Significantly, MFM-115a displays an exceptionally high deliverable CH4 capacity of 208 v/v between 5 and 80 bar at room temperature, making it among the best performing metal–organic frameworks for CH4 storage. We also synthesized the partially deuterated versions of the above materials and applied solid-state 2H NMR spectroscopy to show that these three frameworks contain molecular rotors that exhibit motion in fast, medium, and slow regimes, respectively. In situ neutron powder diffraction studies on the binding sites for CD4 within MFM-132a and MFM-115a reveal that the primary binding site is located within the small pocket enclosed by the [(Cu2)3(isophthalate)3] window and three anthracene/phenyl panels. The open Cu(II) sites are the secondary/tertiary adsorption sites in these structures. Thus, we obtained direct experimental evidence showing that a tight cavity can generate a stronger binding affinity to gas molecules than open metal sites. Solid-state 2H NMR spectroscopy and neutron diffraction studies reveal that it is the combination of optimal molecular dynamics, pore geometry and size, and favorable binding sites that leads to the exceptional and different methane uptakes in these materials.
中文翻译:

具有最佳分子动力学和孔几何形状的甲烷多孔金属有机多面体构架
天然气(甲烷,CH 4)被广泛认为是用于移动应用的有前途的能源载体。最大化存储容量是未来存储介质设计的主要目标。在这里,我们报告了在同构结构(3,24)连接的多孔材料MFM-112a,MFM-115a和MFM-132a系列中具有不同接头骨架功能化的CH 4的存储特性。MFM-112a和MFM-115a在80 bar和室温下均表现出出色的CH 4吸收率,分别为236和256 cm 3(STP)cm -3(v / v)。值得一提的是,MFM-115a的CH 4交付量极高在室温下5至80 bar之间的最大容量为208 v / v,使其成为CH 4储存性能最好的金属有机框架。我们还合成了上述材料的部分氘代形式,并应用了固态2 H NMR光谱法显示,这三个骨架均包含分子转子,分别在快速,中速和慢速状态下表现出运动。对MFM-132a和MFM-115a中CD 4结合位点的原位中子粉末衍射研究表明,主要结合位点位于[[Cu 2)3(间苯二甲酸)3包围的小袋内]窗口和三个蒽/苯基面板。在这些结构中,开放的Cu(II)位是二级/三级吸附位。因此,我们获得了直接的实验证据,表明紧密的空腔比开放的金属位点对气体分子的结合亲和力更强。固态2 H NMR光谱和中子衍射研究表明,正是最佳分子动力学,孔隙几何形状和尺寸以及良好的结合位点的组合,导致这些材料中甲烷的异常吸收量不同。
更新日期:2017-09-19
中文翻译:

具有最佳分子动力学和孔几何形状的甲烷多孔金属有机多面体构架
天然气(甲烷,CH 4)被广泛认为是用于移动应用的有前途的能源载体。最大化存储容量是未来存储介质设计的主要目标。在这里,我们报告了在同构结构(3,24)连接的多孔材料MFM-112a,MFM-115a和MFM-132a系列中具有不同接头骨架功能化的CH 4的存储特性。MFM-112a和MFM-115a在80 bar和室温下均表现出出色的CH 4吸收率,分别为236和256 cm 3(STP)cm -3(v / v)。值得一提的是,MFM-115a的CH 4交付量极高在室温下5至80 bar之间的最大容量为208 v / v,使其成为CH 4储存性能最好的金属有机框架。我们还合成了上述材料的部分氘代形式,并应用了固态2 H NMR光谱法显示,这三个骨架均包含分子转子,分别在快速,中速和慢速状态下表现出运动。对MFM-132a和MFM-115a中CD 4结合位点的原位中子粉末衍射研究表明,主要结合位点位于[[Cu 2)3(间苯二甲酸)3包围的小袋内]窗口和三个蒽/苯基面板。在这些结构中,开放的Cu(II)位是二级/三级吸附位。因此,我们获得了直接的实验证据,表明紧密的空腔比开放的金属位点对气体分子的结合亲和力更强。固态2 H NMR光谱和中子衍射研究表明,正是最佳分子动力学,孔隙几何形状和尺寸以及良好的结合位点的组合,导致这些材料中甲烷的异常吸收量不同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号