当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of a Thioesterase Bottleneck in the Pikromycin Pathway through Full-Module Processing of Unnatural Pentaketides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/jacs.7b06432 Douglas A. Hansen 1 , Aaron A. Koch 1 , David H. Sherman 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/jacs.7b06432 Douglas A. Hansen 1 , Aaron A. Koch 1 , David H. Sherman 1
Affiliation
|
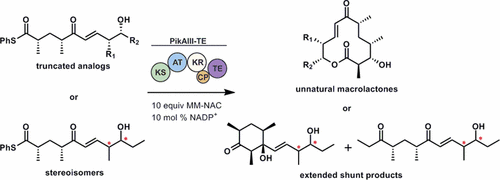
Polyketide biosynthetic pathways have been engineered to generate natural product analogs for over two decades. However, manipulation of modular type I polyketide synthases (PKSs) to make unnatural metabolites commonly results in attenuated yields or entirely inactive pathways, and the mechanistic basis for compromised production is rarely elucidated since rate-limiting or inactive domain(s) remain unidentified. Accordingly, we synthesized and assayed a series of modified pikromycin (Pik) pentaketides that mimic early pathway engineering to probe the substrate tolerance of the PikAIII-TE module in vitro. Truncated pentaketides were processed with varying efficiencies to corresponding macrolactones, while pentaketides with epimerized chiral centers were poorly processed by PikAIII-TE and failed to generate 12-membered ring products. Isolation and identification of extended but prematurely offloaded shunt products suggested that the Pik thioesterase (TE) domain has limited substrate flexibility and functions as a gatekeeper in the processing of unnatural substrates. Synthesis of an analogous hexaketide with an epimerized nucleophilic hydroxyl group allowed for direct evaluation of the substrate stereoselectivity of the excised TE domain. The epimerized hexaketide failed to undergo cyclization and was exclusively hydrolyzed, confirming the TE domain as a key catalytic bottleneck. In an accompanying paper, we engineer the standalone Pik thioesterase to yield a thioesterase (TES148C) and module (PikAIII-TES148C) that display gain-of-function processing of substrates with inverted hydroxyl groups.
中文翻译:

通过非天然Penteptides的全模块处理鉴定吡咯霉素途径中的硫酯酶瓶颈。
聚酮化合物的生物合成途径已被设计用于生成天然产物类似物已有二十多年的历史。然而,操纵模块I型聚酮化合物合酶(PKS)以产生非天然代谢物通常会导致产量降低或完全失活的途径,并且由于速率限制或失活的域仍未确定,因此很难阐明损害产量的机理。因此,我们合成并分析了一系列修饰的吡咯霉素(Pik)五肽,它们模仿了早期途径工程,以体外探查PikAIII-TE模块的底物耐受性。截短的五肽可能以不同的效率处理相应的大内酯,而带有差向异构手性中心的五肽被PikAIII-TE处理得很差,无法生成12元环产品。扩展和过早分流产品的分离和鉴定表明,Pik硫酯酶(TE)结构域具有有限的底物灵活性,并在处理非天然底物时起着关守的作用。具有差向异构的亲核羟基的类似六酮化合物的合成,可以直接评估被切除的TE结构域的底物立体选择性。差向异构化的六酮化合物未能进行环化,仅被水解,从而确认TE结构域是关键的催化瓶颈。在 具有差向异构的亲核羟基的类似六酮化合物的合成,可以直接评估被切除的TE结构域的底物立体选择性。差向异构化的六酮化合物未能进行环化,仅被水解,从而确认TE结构域是关键的催化瓶颈。在 具有差向异构的亲核羟基的类似六酮化合物的合成,可以直接评估被切除的TE结构域的底物立体选择性。差向异构化的六酮化合物未能进行环化,仅被水解,从而确认TE结构域是关键的催化瓶颈。在在随附的论文中,我们对独立的Pik硫酯酶进行了工程改造,以产生硫酯酶(TE S148C)和模块(PikAIII-TE S148C),该模块显示了具有反向羟基的底物的功能增益处理。
更新日期:2017-09-19
中文翻译:

通过非天然Penteptides的全模块处理鉴定吡咯霉素途径中的硫酯酶瓶颈。
聚酮化合物的生物合成途径已被设计用于生成天然产物类似物已有二十多年的历史。然而,操纵模块I型聚酮化合物合酶(PKS)以产生非天然代谢物通常会导致产量降低或完全失活的途径,并且由于速率限制或失活的域仍未确定,因此很难阐明损害产量的机理。因此,我们合成并分析了一系列修饰的吡咯霉素(Pik)五肽,它们模仿了早期途径工程,以体外探查PikAIII-TE模块的底物耐受性。截短的五肽可能以不同的效率处理相应的大内酯,而带有差向异构手性中心的五肽被PikAIII-TE处理得很差,无法生成12元环产品。扩展和过早分流产品的分离和鉴定表明,Pik硫酯酶(TE)结构域具有有限的底物灵活性,并在处理非天然底物时起着关守的作用。具有差向异构的亲核羟基的类似六酮化合物的合成,可以直接评估被切除的TE结构域的底物立体选择性。差向异构化的六酮化合物未能进行环化,仅被水解,从而确认TE结构域是关键的催化瓶颈。在 具有差向异构的亲核羟基的类似六酮化合物的合成,可以直接评估被切除的TE结构域的底物立体选择性。差向异构化的六酮化合物未能进行环化,仅被水解,从而确认TE结构域是关键的催化瓶颈。在 具有差向异构的亲核羟基的类似六酮化合物的合成,可以直接评估被切除的TE结构域的底物立体选择性。差向异构化的六酮化合物未能进行环化,仅被水解,从而确认TE结构域是关键的催化瓶颈。在在随附的论文中,我们对独立的Pik硫酯酶进行了工程改造,以产生硫酯酶(TE S148C)和模块(PikAIII-TE S148C),该模块显示了具有反向羟基的底物的功能增益处理。









































 京公网安备 11010802027423号
京公网安备 11010802027423号