当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical Reduction of Carbon Dioxide in a Monoethanolamine Capture Medium
ChemSusChem ( IF 8.4 ) Pub Date : 2017-09-19 07:25:26 , DOI: 10.1002/cssc.201701075 Lu Chen 1 , Fengwang Li 1 , Ying Zhang 1 , Cameron L. Bentley 2 , Mike Horne 3 , Alan M. Bond 1 , Jie Zhang 1
ChemSusChem ( IF 8.4 ) Pub Date : 2017-09-19 07:25:26 , DOI: 10.1002/cssc.201701075 Lu Chen 1 , Fengwang Li 1 , Ying Zhang 1 , Cameron L. Bentley 2 , Mike Horne 3 , Alan M. Bond 1 , Jie Zhang 1
Affiliation
|
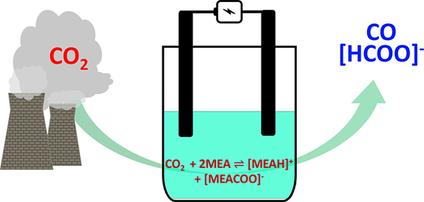
Captured for reduction: The electrocatalytic reduction of CO2 in a monoethanolamine capture medium has been undertaken at In, Sn, Bi, Pb, Pd, Ag, Cu, and Zn metal electrodes. Both an increase in the electrode surface porosity and the addition of the surfactant cetyltrimethylammonium bromide suppress the competing hydrogen evolution reaction and, therefore, produce much improved faradaic efficiencies for CO2 reduction.
中文翻译:

单乙醇胺捕获介质中二氧化碳的电化学还原
为还原而捕获:在In,Sn,Bi,Pb,Pd,Ag,Cu和Zn金属电极上,已在单乙醇胺捕获介质中对CO 2进行了电催化还原。电极表面孔隙率的增加和表面活性剂十六烷基三甲基溴化铵的添加都抑制了竞争性氢释放反应,因此,产生了大大提高的法拉第效率以降低CO 2。
更新日期:2017-09-19
中文翻译:

单乙醇胺捕获介质中二氧化碳的电化学还原
为还原而捕获:在In,Sn,Bi,Pb,Pd,Ag,Cu和Zn金属电极上,已在单乙醇胺捕获介质中对CO 2进行了电催化还原。电极表面孔隙率的增加和表面活性剂十六烷基三甲基溴化铵的添加都抑制了竞争性氢释放反应,因此,产生了大大提高的法拉第效率以降低CO 2。



























 京公网安备 11010802027423号
京公网安备 11010802027423号