当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analogues of the 2-carboxyl-6-hydroxyoctahydroindole (CHOI) unit from diverging Pd-catalyzed allylations: Selectivity as a function of the double bond position
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.tetlet.2017.09.046 Zhongyi Mao , Elisabetta Martini , Guillaume Prestat , Julie Oble , Pei-Qiang Huang , Giovanni Poli
中文翻译:

分散的Pd催化的烯丙基化过程中的2-羧基-6-羟基八氢吲哚(CHOI)单元的类似物:选择性与双键位置的关系
更新日期:2017-09-18
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.tetlet.2017.09.046 Zhongyi Mao , Elisabetta Martini , Guillaume Prestat , Julie Oble , Pei-Qiang Huang , Giovanni Poli
|
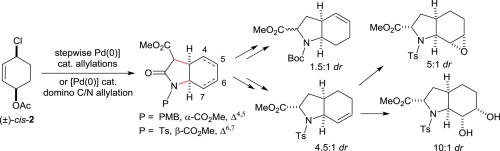
Pd-catalyzed allylations of cyclic bis-allylic substrates, carried out either as two separate steps or in a pseudo-domino fashion, can generate 2-carboxyl-hexahydroindoles bearing an unsaturation in different positions. Sequential homologation, and epoxidation or syn-dihydroxylation steps were investigated to access analogues of the bicyclic 2-carboxyl-6-hydroxyoctahydroindole motif of aeruginosins, a family of peptides displaying serine protease inhibitor activity.
中文翻译:

分散的Pd催化的烯丙基化过程中的2-羧基-6-羟基八氢吲哚(CHOI)单元的类似物:选择性与双键位置的关系
钯催化的环状双烯丙基底物的烯丙基化,以两个单独的步骤或以假多米诺方式进行,可以生成在不同位置带有不饱和键的2-羧基-六氢吲哚。为了获得铜绿素酶的双环2-羧基-6-羟基八氢吲哚基序的序列类似物,环氧化或顺-二羟基化步骤进行了研究,这是一个显示丝氨酸蛋白酶抑制剂活性的肽家族。











































 京公网安备 11010802027423号
京公网安备 11010802027423号