Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Lorazepam With Haloperidol vs Haloperidol Alone on Agitated Delirium in Patients With Advanced Cancer Receiving Palliative Care
JAMA ( IF 63.1 ) Pub Date : 2017-09-19 , DOI: 10.1001/jama.2017.11468 David Hui 1 , Susan Frisbee-Hume 1 , Annie Wilson 1 , Seyedeh S Dibaj 2 , Thuc Nguyen 1 , Maxine De La Cruz 1 , Paul Walker 1 , Donna S Zhukovsky 1 , Marvin Delgado-Guay 1 , Marieberta Vidal 1 , Daniel Epner 1 , Akhila Reddy 1 , Kimerson Tanco 1 , Janet Williams 1 , Stacy Hall 1 , Diane Liu 2 , Kenneth Hess 2 , Sapna Amin 3 , William Breitbart 4 , Eduardo Bruera 1
JAMA ( IF 63.1 ) Pub Date : 2017-09-19 , DOI: 10.1001/jama.2017.11468 David Hui 1 , Susan Frisbee-Hume 1 , Annie Wilson 1 , Seyedeh S Dibaj 2 , Thuc Nguyen 1 , Maxine De La Cruz 1 , Paul Walker 1 , Donna S Zhukovsky 1 , Marvin Delgado-Guay 1 , Marieberta Vidal 1 , Daniel Epner 1 , Akhila Reddy 1 , Kimerson Tanco 1 , Janet Williams 1 , Stacy Hall 1 , Diane Liu 2 , Kenneth Hess 2 , Sapna Amin 3 , William Breitbart 4 , Eduardo Bruera 1
Affiliation
|
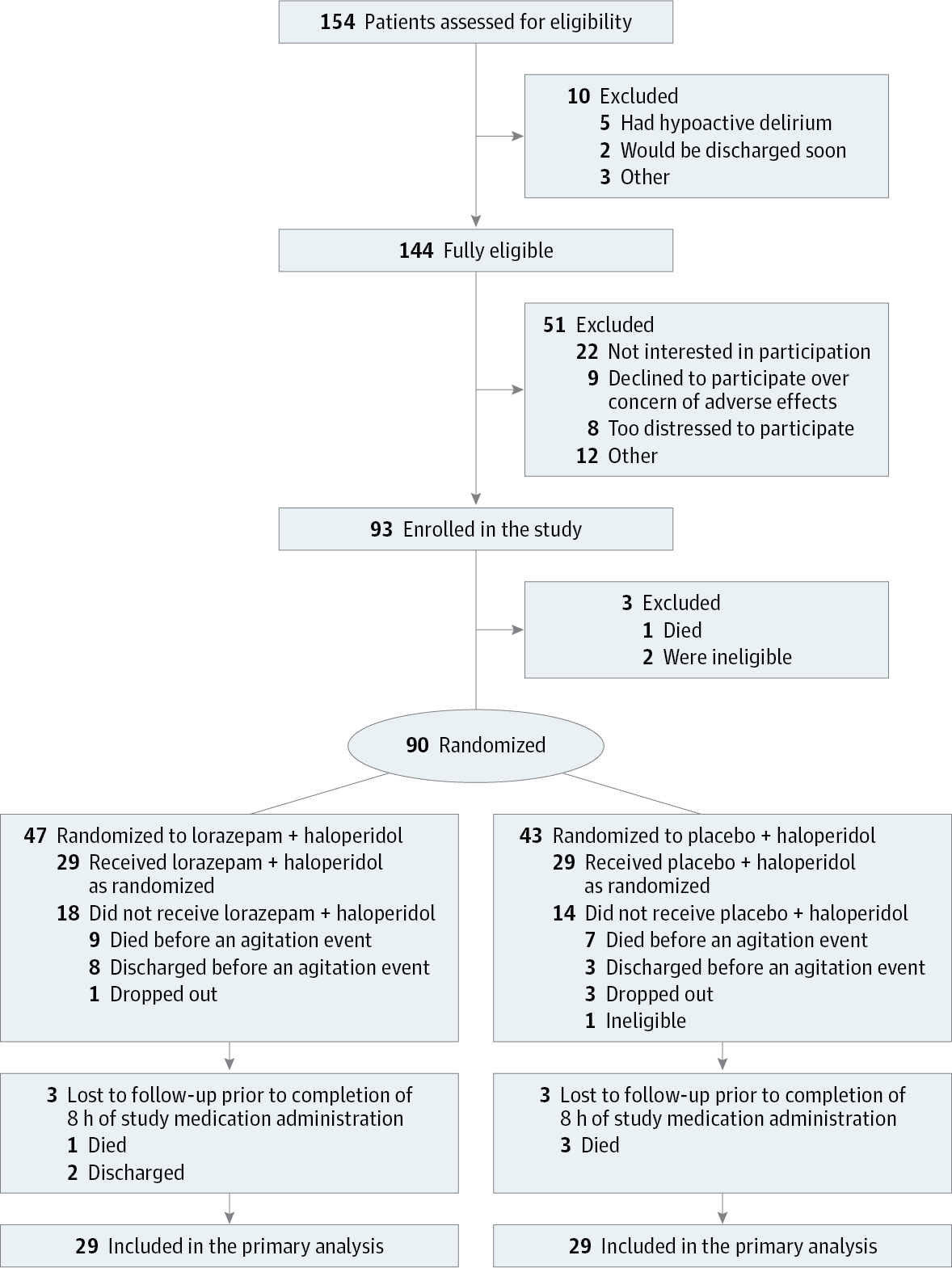
Importance The use of benzodiazepines to control agitation in delirium in the last days of life is controversial. Objective To compare the effect of lorazepam vs placebo as an adjuvant to haloperidol for persistent agitation in patients with delirium in the setting of advanced cancer. Design, Setting, and Participants Single-center, double-blind, parallel-group, randomized clinical trial conducted at an acute palliative care unit at MD Anderson Cancer Center, Texas, enrolling 93 patients with advanced cancer and agitated delirium despite scheduled haloperidol from February 11, 2014, to June 30, 2016, with data collection completed in October 2016. Interventions Lorazepam (3 mg) intravenously (n = 47) or placebo (n = 43) in addition to haloperidol (2 mg) intravenously upon the onset of an agitation episode. Main Outcomes and Measures The primary outcome was change in Richmond Agitation-Sedation Scale (RASS) score (range, −5 [unarousable] to 4 [very agitated or combative]) from baseline to 8 hours after treatment administration. Secondary end points were rescue neuroleptic use, delirium recall, comfort (perceived by caregivers and nurses), communication capacity, delirium severity, adverse effects, discharge outcomes, and overall survival. Results Among 90 randomized patients (mean age, 62 years; women, 42 [47%]), 58 (64%) received the study medication and 52 (90%) completed the trial. Lorazepam + haloperidol resulted in a significantly greater reduction of RASS score at 8 hours (−4.1 points) than placebo + haloperidol (−2.3 points) (mean difference, −1.9 points [95% CI, −2.8 to −0.9]; P < .001). The lorazepam + haloperidol group required less median rescue neuroleptics (2.0 mg) than the placebo + haloperidol group (4.0 mg) (median difference, −1.0 mg [95% CI, −2.0 to 0]; P = .009) and was perceived to be more comfortable by both blinded caregivers and nurses (caregivers: 84% for the lorazepam + haloperidol group vs 37% for the placebo + haloperidol group; mean difference, 47% [95% CI, 14% to 73%], P = .007; nurses: 77% for the lorazepam + haloperidol group vs 30% for the placebo + haloperidol group; mean difference, 47% [95% CI, 17% to 71%], P = .005). No significant between-group differences were found in delirium-related distress and survival. The most common adverse effect was hypokinesia (3 patients in the lorazepam + haloperidol group [19%] and 4 patients in the placebo + haloperidol group [27%]). Conclusions and Relevance In this preliminary trial of hospitalized patients with agitated delirium in the setting of advanced cancer, the addition of lorazepam to haloperidol compared with haloperidol alone resulted in a significantly greater reduction in agitation at 8 hours. Further research is needed to assess generalizability and adverse effects. Trial Registration clinicaltrials.gov Identifier: NCT01949662
中文翻译:

劳拉西泮联合氟哌啶醇与单独氟哌啶醇对接受姑息治疗的晚期癌症患者躁动性谵妄的影响
重要性 在生命最后几天使用苯二氮卓类药物来控制谵妄状态下的躁动是有争议的。目的 比较劳拉西泮与安慰剂作为氟哌啶醇佐剂治疗晚期癌症谵妄患者持续性躁动的效果。设计、设置和参与者 单中心、双盲、平行组、随机临床试验在德克萨斯州 MD 安德森癌症中心的急性姑息治疗病房进行,入组了 93 名晚期癌症患者,尽管从 2 月份开始已安排氟哌啶醇治疗,但仍出现躁动性谵妄症状2014年11月11日至2016年6月30日,数据收集于2016年10月完成。 干预措施 发病时静脉注射劳拉西泮(3 mg)(n = 47)或安慰剂(n = 43)以及静脉注射氟哌啶醇(2 mg)激动人心的一幕主要结果和措施 主要结果是里士满激动-镇静量表 (RASS) 评分(范围,-5 [无法激动] 至 4 [非常激动或好斗])从基线到治疗后 8 小时的变化。次要终点是救援抗精神病药的使用、谵妄回忆、舒适度(护理人员和护士感受到的)、沟通能力、谵妄严重程度、不良反应、出院结果和总生存期。结果 在 90 名随机患者(平均年龄 62 岁;女性 42 名 [47%])中,58 名 (64%) 接受了研究药物治疗,52 名 (90%) 完成了试验。与安慰剂 + 氟哌啶醇 (-2.3 分) 相比,劳拉西泮 + 氟哌啶醇在 8 小时时导致 RASS 评分显着降低 (-4.1 分)(平均差异,-1.9 分 [95% CI,-2.8 至 -0.9];P < .001)。劳拉西泮 + 氟哌啶醇组需要的中位救援精神安定药 (2.0 mg) 少于安慰剂 + 氟哌啶醇组 (4.0 mg)(中位差异,-1.0 mg [95% CI, -2.0 到 0]; P = .009),盲法护理人员和护士均认为感觉更舒适(护理人员:劳拉西泮 + 氟哌啶醇组为 84%,安慰剂 + 氟哌啶醇组为 37%;平均差异,47% [95% CI, 14] % 至 73%],P = .007;护士:劳拉西泮 + 氟哌啶醇组为 77%,安慰剂 + 氟哌啶醇组为 30%;平均差异,47% [95% CI,17% 至 71%],P = .005)。在谵妄相关的痛苦和生存方面没有发现显着的组间差异。最常见的不良反应是运动功能减退(劳拉西泮 + 氟哌啶醇组有 3 名患者 [19%],安慰剂 + 氟哌啶醇组有 4 名患者 [27%])。结论和相关性 在这项针对患有晚期癌症的躁动性谵妄住院患者的初步试验中,与单独使用氟哌啶醇相比,在氟哌啶醇中添加劳拉西泮可在 8 小时内显着更大程度地减少躁动。需要进一步的研究来评估普遍性和不利影响。试验注册临床试验。政府标识符:NCT01949662
更新日期:2017-09-19
中文翻译:

劳拉西泮联合氟哌啶醇与单独氟哌啶醇对接受姑息治疗的晚期癌症患者躁动性谵妄的影响
重要性 在生命最后几天使用苯二氮卓类药物来控制谵妄状态下的躁动是有争议的。目的 比较劳拉西泮与安慰剂作为氟哌啶醇佐剂治疗晚期癌症谵妄患者持续性躁动的效果。设计、设置和参与者 单中心、双盲、平行组、随机临床试验在德克萨斯州 MD 安德森癌症中心的急性姑息治疗病房进行,入组了 93 名晚期癌症患者,尽管从 2 月份开始已安排氟哌啶醇治疗,但仍出现躁动性谵妄症状2014年11月11日至2016年6月30日,数据收集于2016年10月完成。 干预措施 发病时静脉注射劳拉西泮(3 mg)(n = 47)或安慰剂(n = 43)以及静脉注射氟哌啶醇(2 mg)激动人心的一幕主要结果和措施 主要结果是里士满激动-镇静量表 (RASS) 评分(范围,-5 [无法激动] 至 4 [非常激动或好斗])从基线到治疗后 8 小时的变化。次要终点是救援抗精神病药的使用、谵妄回忆、舒适度(护理人员和护士感受到的)、沟通能力、谵妄严重程度、不良反应、出院结果和总生存期。结果 在 90 名随机患者(平均年龄 62 岁;女性 42 名 [47%])中,58 名 (64%) 接受了研究药物治疗,52 名 (90%) 完成了试验。与安慰剂 + 氟哌啶醇 (-2.3 分) 相比,劳拉西泮 + 氟哌啶醇在 8 小时时导致 RASS 评分显着降低 (-4.1 分)(平均差异,-1.9 分 [95% CI,-2.8 至 -0.9];P < .001)。劳拉西泮 + 氟哌啶醇组需要的中位救援精神安定药 (2.0 mg) 少于安慰剂 + 氟哌啶醇组 (4.0 mg)(中位差异,-1.0 mg [95% CI, -2.0 到 0]; P = .009),盲法护理人员和护士均认为感觉更舒适(护理人员:劳拉西泮 + 氟哌啶醇组为 84%,安慰剂 + 氟哌啶醇组为 37%;平均差异,47% [95% CI, 14] % 至 73%],P = .007;护士:劳拉西泮 + 氟哌啶醇组为 77%,安慰剂 + 氟哌啶醇组为 30%;平均差异,47% [95% CI,17% 至 71%],P = .005)。在谵妄相关的痛苦和生存方面没有发现显着的组间差异。最常见的不良反应是运动功能减退(劳拉西泮 + 氟哌啶醇组有 3 名患者 [19%],安慰剂 + 氟哌啶醇组有 4 名患者 [27%])。结论和相关性 在这项针对患有晚期癌症的躁动性谵妄住院患者的初步试验中,与单独使用氟哌啶醇相比,在氟哌啶醇中添加劳拉西泮可在 8 小时内显着更大程度地减少躁动。需要进一步的研究来评估普遍性和不利影响。试验注册临床试验。政府标识符:NCT01949662











































 京公网安备 11010802027423号
京公网安备 11010802027423号