当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure-Guided Screening for Functionally Selective D2 Dopamine Receptor Ligands from a Virtual Chemical Library
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acschembio.7b00493 Barbara Männel 1 , Mariama Jaiteh 2 , Alexey Zeifman 2 , Alena Randakova 1 , Dorothee Möller 1 , Harald Hübner 1 , Peter Gmeiner 1 , Jens Carlsson 2
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acschembio.7b00493 Barbara Männel 1 , Mariama Jaiteh 2 , Alexey Zeifman 2 , Alena Randakova 1 , Dorothee Möller 1 , Harald Hübner 1 , Peter Gmeiner 1 , Jens Carlsson 2
Affiliation
|
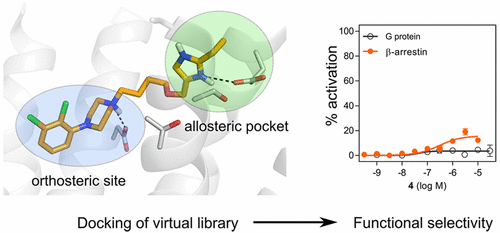
Functionally selective ligands stabilize conformations of G protein-coupled receptors (GPCRs) that induce a preference for signaling via a subset of the intracellular pathways activated by the endogenous agonists. The possibility to fine-tune the functional activity of a receptor provides opportunities to develop drugs that selectively signal via pathways associated with a therapeutic effect and avoid those causing side effects. Animal studies have indicated that ligands displaying functional selectivity at the D2 dopamine receptor (D2R) could be safer and more efficacious drugs against neuropsychiatric diseases. In this work, computational design of functionally selective D2R ligands was explored using structure-based virtual screening. Molecular docking of known functionally selective ligands to a D2R homology model indicated that such compounds were anchored by interactions with the orthosteric site and extended into a common secondary pocket. A tailored virtual library with close to 13 000 compounds bearing 2,3-dichlorophenylpiperazine, a privileged orthosteric scaffold, connected to diverse chemical moieties via a linker was docked to the D2R model. Eighteen top-ranked compounds that occupied both the orthosteric and allosteric site were synthesized, leading to the discovery of 16 partial agonists. A majority of the ligands had comparable maximum effects in the G protein and β-arrestin recruitment assays, but a subset displayed preference for a single pathway. In particular, compound 4 stimulated β-arrestin recruitment (EC50 = 320 nM, Emax = 16%) but had no detectable G protein signaling. The use of structure-based screening and virtual libraries to discover GPCR ligands with tailored functional properties will be discussed.
中文翻译:

虚拟化学文库中功能选择性D 2多巴胺受体配体的结构指导筛选
功能选择性配体稳定诱导用于信令的偏好G蛋白偶联受体(GPCR)的构象通过由内源性激动剂激活胞内途径的子集。微调受体功能活性的可能性为开发药物提供了机会,这些药物可以通过与治疗作用相关的途径选择性地发出信号,并避免那些引起副作用的途径。动物研究表明,在D 2多巴胺受体(D 2 R)上显示功能选择性的配体可能是更安全有效的抗神经精神疾病药物。在这项工作中,功能选择D 2的计算设计使用基于结构的虚拟筛选探索R配体。已知的功能选择性配体与D 2 R同源性模型的分子对接表明,此类化合物通过与正构位点的相互作用而锚定,并延伸到共同的第二个口袋中。定制的虚拟文库与D 2停靠在一起,该虚拟文库将近13000个带有2,3-二氯苯基哌嗪(一种特有的正构支架)的化合物,该化合物通过连接器连接到各种化学部分,是一种特有的正构支架。R模型。合成了18个占据正构和变构位点的顶级化合物,从而发现了16个部分激动剂。大多数配体在G蛋白和β-arrestin募集试验中具有可比的最大作用,但有一部分显示出对单途径的偏爱。特别是,化合物4刺激了β-arrestin募集(EC 50 = 320 nM,E max = 16%),但没有可检测的G蛋白信号传导。将讨论使用基于结构的筛选和虚拟文库来发现具有定制功能特性的GPCR配体。
更新日期:2017-09-19
中文翻译:

虚拟化学文库中功能选择性D 2多巴胺受体配体的结构指导筛选
功能选择性配体稳定诱导用于信令的偏好G蛋白偶联受体(GPCR)的构象通过由内源性激动剂激活胞内途径的子集。微调受体功能活性的可能性为开发药物提供了机会,这些药物可以通过与治疗作用相关的途径选择性地发出信号,并避免那些引起副作用的途径。动物研究表明,在D 2多巴胺受体(D 2 R)上显示功能选择性的配体可能是更安全有效的抗神经精神疾病药物。在这项工作中,功能选择D 2的计算设计使用基于结构的虚拟筛选探索R配体。已知的功能选择性配体与D 2 R同源性模型的分子对接表明,此类化合物通过与正构位点的相互作用而锚定,并延伸到共同的第二个口袋中。定制的虚拟文库与D 2停靠在一起,该虚拟文库将近13000个带有2,3-二氯苯基哌嗪(一种特有的正构支架)的化合物,该化合物通过连接器连接到各种化学部分,是一种特有的正构支架。R模型。合成了18个占据正构和变构位点的顶级化合物,从而发现了16个部分激动剂。大多数配体在G蛋白和β-arrestin募集试验中具有可比的最大作用,但有一部分显示出对单途径的偏爱。特别是,化合物4刺激了β-arrestin募集(EC 50 = 320 nM,E max = 16%),但没有可检测的G蛋白信号传导。将讨论使用基于结构的筛选和虚拟文库来发现具有定制功能特性的GPCR配体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号