当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative structure–activity relationships for reactivities of sulfate and hydroxyl radicals with aromatic contaminants through single–electron transfer pathway
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.jhazmat.2017.09.024 Shuang Luo , Zongsu Wei , Richard Spinney , Frederick A. Villamena , Dionysios D. Dionysiou , Dong Chen , Chong-Jian Tang , Liyuan Chai , Ruiyang Xiao
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.jhazmat.2017.09.024 Shuang Luo , Zongsu Wei , Richard Spinney , Frederick A. Villamena , Dionysios D. Dionysiou , Dong Chen , Chong-Jian Tang , Liyuan Chai , Ruiyang Xiao
|
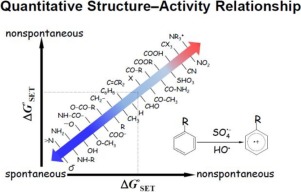
Sulfate radical anion () and hydroxyl radical ( OH) based advanced oxidation technologies has been extensively used for removal of aromatic contaminants (ACs) in waters. In this study, we investigated the Gibbs free energy (
OH) based advanced oxidation technologies has been extensively used for removal of aromatic contaminants (ACs) in waters. In this study, we investigated the Gibbs free energy ( ) of the single electron transfer (SET) reactions for 76 ACs with
) of the single electron transfer (SET) reactions for 76 ACs with  and
and  OH, respectively. The result reveals that
OH, respectively. The result reveals that  possesses greater propensity to react with ACs through the SET channel than
possesses greater propensity to react with ACs through the SET channel than  OH. We hypothesized that the electron distribution within the molecule plays an essential role in determining the
OH. We hypothesized that the electron distribution within the molecule plays an essential role in determining the  and subsequent SET reactions. To test the hypothesis, a quantitative structure−activity relationship (QSAR) model was developed for predicting
and subsequent SET reactions. To test the hypothesis, a quantitative structure−activity relationship (QSAR) model was developed for predicting  using the highest occupied molecular orbital energies (EHOMO), a measure of electron distribution and donating ability. The standardized QSAR models are reported to be ΔG°SET = −0.97 × EHOMO − 181 and ΔG°SET = −0.97 × EHOMO − 164 for
using the highest occupied molecular orbital energies (EHOMO), a measure of electron distribution and donating ability. The standardized QSAR models are reported to be ΔG°SET = −0.97 × EHOMO − 181 and ΔG°SET = −0.97 × EHOMO − 164 for  and
and  OH, respectively. The models were internally and externally validated to ensure robustness and predictability, and the application domain and limitations were discussed. The single–descriptor based models account for 95% of the variability for
OH, respectively. The models were internally and externally validated to ensure robustness and predictability, and the application domain and limitations were discussed. The single–descriptor based models account for 95% of the variability for  and
and  OH. These results provide the mechanistic insight into the SET reaction pathway of radical and non–radical bimolecular reactions, and have important applications for radical based oxidation technologies to remove target ACs in different waters.
OH. These results provide the mechanistic insight into the SET reaction pathway of radical and non–radical bimolecular reactions, and have important applications for radical based oxidation technologies to remove target ACs in different waters.
中文翻译:

通过单电子转移途径与芳香族污染物反应的硫酸根和羟基自由基的定量构效关系
硫酸根阴离子()和羟基( 基于OH)的先进氧化技术已被广泛用于去除水中的芳香族污染物(AC)。在这项研究中,我们研究了吉布斯自由能(
基于OH)的先进氧化技术已被广泛用于去除水中的芳香族污染物(AC)。在这项研究中,我们研究了吉布斯自由能( )的76个AC的单电子转移(SET)反应
)的76个AC的单电子转移(SET)反应  和
和  分别。结果表明
分别。结果表明 与通过SET通道与AC进行反应相比,具有更大的倾向
与通过SET通道与AC进行反应相比,具有更大的倾向  哦。我们假设分子内的电子分布在确定分子的内在起着至关重要的作用。
哦。我们假设分子内的电子分布在确定分子的内在起着至关重要的作用。 以及随后的SET反应。为了检验假设,开发了定量构效关系(QSAR)模型来预测
以及随后的SET反应。为了检验假设,开发了定量构效关系(QSAR)模型来预测 使用最高的分子轨道能量(E HOMO)来衡量电子分布和供电能力。据报道,对于以下情况,标准化的QSAR模型为ΔG ° SET = −0.97× E HOMO − 181和ΔG ° SET = −0.97× E HOMO − 164
使用最高的分子轨道能量(E HOMO)来衡量电子分布和供电能力。据报道,对于以下情况,标准化的QSAR模型为ΔG ° SET = −0.97× E HOMO − 181和ΔG ° SET = −0.97× E HOMO − 164 和
和  分别。对模型进行了内部和外部验证,以确保鲁棒性和可预测性,并讨论了应用领域和局限性。基于单描述符的模型占据了95%的可变性
分别。对模型进行了内部和外部验证,以确保鲁棒性和可预测性,并讨论了应用领域和局限性。基于单描述符的模型占据了95%的可变性 和
和  哦。这些结果提供了对自由基和非自由基双分子反应的SET反应途径的机理认识,并在基于自由基的氧化技术中去除不同水中的目标AC方面具有重要的应用。
哦。这些结果提供了对自由基和非自由基双分子反应的SET反应途径的机理认识,并在基于自由基的氧化技术中去除不同水中的目标AC方面具有重要的应用。
更新日期:2017-09-19
 OH) based advanced oxidation technologies has been extensively used for removal of aromatic contaminants (ACs) in waters. In this study, we investigated the Gibbs free energy (
OH) based advanced oxidation technologies has been extensively used for removal of aromatic contaminants (ACs) in waters. In this study, we investigated the Gibbs free energy ( OH, respectively. The result reveals that
OH, respectively. The result reveals that  OH. We hypothesized that the electron distribution within the molecule plays an essential role in determining the
OH. We hypothesized that the electron distribution within the molecule plays an essential role in determining the  OH, respectively. The models were internally and externally validated to ensure robustness and predictability, and the application domain and limitations were discussed. The single–descriptor based models account for 95% of the variability for
OH, respectively. The models were internally and externally validated to ensure robustness and predictability, and the application domain and limitations were discussed. The single–descriptor based models account for 95% of the variability for  OH. These results provide the mechanistic insight into the SET reaction pathway of radical and non–radical bimolecular reactions, and have important applications for radical based oxidation technologies to remove target ACs in different waters.
OH. These results provide the mechanistic insight into the SET reaction pathway of radical and non–radical bimolecular reactions, and have important applications for radical based oxidation technologies to remove target ACs in different waters.
中文翻译:

通过单电子转移途径与芳香族污染物反应的硫酸根和羟基自由基的定量构效关系
硫酸根阴离子()和羟基(
 基于OH)的先进氧化技术已被广泛用于去除水中的芳香族污染物(AC)。在这项研究中,我们研究了吉布斯自由能(
基于OH)的先进氧化技术已被广泛用于去除水中的芳香族污染物(AC)。在这项研究中,我们研究了吉布斯自由能( 分别。结果表明
分别。结果表明 哦。我们假设分子内的电子分布在确定分子的内在起着至关重要的作用。
哦。我们假设分子内的电子分布在确定分子的内在起着至关重要的作用。 分别。对模型进行了内部和外部验证,以确保鲁棒性和可预测性,并讨论了应用领域和局限性。基于单描述符的模型占据了95%的可变性
分别。对模型进行了内部和外部验证,以确保鲁棒性和可预测性,并讨论了应用领域和局限性。基于单描述符的模型占据了95%的可变性 哦。这些结果提供了对自由基和非自由基双分子反应的SET反应途径的机理认识,并在基于自由基的氧化技术中去除不同水中的目标AC方面具有重要的应用。
哦。这些结果提供了对自由基和非自由基双分子反应的SET反应途径的机理认识,并在基于自由基的氧化技术中去除不同水中的目标AC方面具有重要的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号