当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Switchable synthesis of furfurylamine and tetrahydrofurfurylamine from furfuryl alcohol over RANEY® nickel
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-08-17 00:00:00 , DOI: 10.1039/c7cy00981j Yingxin Liu 1, 2, 3, 4, 5 , Kuo Zhou 1, 2, 3, 4, 5 , Huimin Shu 1, 2, 3, 4, 5 , Haiyan Liu 1, 2, 3, 4, 5 , Jiongtao Lou 5, 6, 7, 8, 9 , Dechao Guo 5, 6, 7, 8, 9 , Zuojun Wei 5, 6, 7, 8, 9 , Xiaonian Li 3, 4, 5, 10, 11
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-08-17 00:00:00 , DOI: 10.1039/c7cy00981j Yingxin Liu 1, 2, 3, 4, 5 , Kuo Zhou 1, 2, 3, 4, 5 , Huimin Shu 1, 2, 3, 4, 5 , Haiyan Liu 1, 2, 3, 4, 5 , Jiongtao Lou 5, 6, 7, 8, 9 , Dechao Guo 5, 6, 7, 8, 9 , Zuojun Wei 5, 6, 7, 8, 9 , Xiaonian Li 3, 4, 5, 10, 11
Affiliation
|
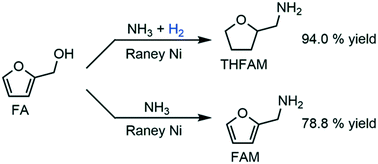
RANEY® Ni proved to be an effective heterogeneous catalyst for switchable reductive amination of furfuryl alcohol to tetrahydrofurfurylamine and furfurylamine with NH3 by simply adding or not adding 1.0 MPa H2 into the reaction bulk. After further optimization of the reaction conditions, we finally obtained 94.0% yield of tetrahydrofurfurylamine and 78.8% yield of furfurylamine with high selectivity. By extensively studying the catalytic pathways and mechanism of catalyst deactivation with XRD and XPS characterization, we have confirmed that an excess amount of H2 in the reaction bulk leads to the deep hydrogenation of the furan ring while an insufficient amount of H2 leads to the formation of Ni3N and the deactivation of the catalyst.
中文翻译:

在RANEY®镍上由糠醇可转换合成糠胺和四氢糠胺
通过简单地在反应物料中添加或不添加1.0 MPa H 2,证明RANEY®Ni是一种有效的非均相催化剂,可用于糠醇可转换还原胺化为NH 3的四氢糠胺和糠胺。在进一步优化反应条件后,我们最终以高选择性获得了94.0%的四氢糠胺和78.8%的糠胺收率。通过广泛研究XRD和XPS表征的催化途径和催化剂失活的机理,我们已经确认,反应主体中过量的H 2导致呋喃环的深度氢化,而不足量的H 2导致呋喃环的加氢。Ni 3的形成N和催化剂失活。
更新日期:2017-09-19
中文翻译:

在RANEY®镍上由糠醇可转换合成糠胺和四氢糠胺
通过简单地在反应物料中添加或不添加1.0 MPa H 2,证明RANEY®Ni是一种有效的非均相催化剂,可用于糠醇可转换还原胺化为NH 3的四氢糠胺和糠胺。在进一步优化反应条件后,我们最终以高选择性获得了94.0%的四氢糠胺和78.8%的糠胺收率。通过广泛研究XRD和XPS表征的催化途径和催化剂失活的机理,我们已经确认,反应主体中过量的H 2导致呋喃环的深度氢化,而不足量的H 2导致呋喃环的加氢。Ni 3的形成N和催化剂失活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号