当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of CO2 on the deactivation behaviors of Co/Al2O3 and Co/SiO2 in CO hydrogenation to hydrocarbons
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1039/c7cy01065f Kyung Soo Park 1, 2, 3 , K. Saravanan 1, 2, 3 , Seon-Ju Park 3, 4, 5 , Yun-Jo Lee 3, 4, 5 , Ki-Won Jeon 3, 4, 5 , Jong Wook Bae 1, 2, 3
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1039/c7cy01065f Kyung Soo Park 1, 2, 3 , K. Saravanan 1, 2, 3 , Seon-Ju Park 3, 4, 5 , Yun-Jo Lee 3, 4, 5 , Ki-Won Jeon 3, 4, 5 , Jong Wook Bae 1, 2, 3
Affiliation
|
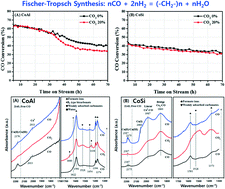
Different deactivation behaviors of the prototype Co/γ-Al2O3 (CoAl) and Co/SiO2 (CoSi) catalysts under an excess CO2 environment were investigated in terms of the surface oxidation and aggregation of cobalt crystallites for the Fischer–Tropsch synthesis (FTS) reaction. The presence of excess CO2 in the syngas feed largely altered the catalytic activity and product distribution, especially on the CoAl catalyst. A relatively faster deactivation and lower C5+ selectivity under an excess CO2 environment were observed on CoAl compared with the CoSi, which also were fairly stable under the same reaction conditions. From measurements of the reduction–oxidation behaviors of the cobalt crystallites, it could be seen that CO2 molecules acted as a mild oxidant by partially oxidizing the exposed metallic cobalt surfaces. The dramatic decrease in CO conversion and an increase in CH4 selectivity under the CO2 environment over CoAl were mainly attributed to the irreducible oxidation of the metallic cobalt surfaces through strong interactions with the Al2O3 support. Meanwhile, the marginal deactivation rate and lower changes of selectivity on CoSi were mainly attributed to the reversible oxidation–reduction property of the metallic cobalt crystallites by forming larger cobalt crystallites with relatively weak interactions with the SiO2 support. An excess exposure to the mild oxidant of CO2 on the FTS catalysts generally decreased the catalytic activity irreversibly by forming strongly interacted large cobalt crystallites, which was more predominantly seen on the acidic Co/Al2O3 than on the Co/SiO2 catalyst. The easy reversibility of the oxidation–reduction of the surface metallic cobalt crystallites on SiO2 even in the presence of excess CO2 could prevent catalyst deactivation during the FTS reaction.
中文翻译:

CO 2对CO加氢制烃过程中Co / Al 2 O 3和Co / SiO 2失活行为的影响
原型的不同失活行为的Co /γ-Al系2 ö 3(煤)和Co /二氧化硅2过量CO下(硅化钴)的催化剂2环境中在表面氧化和钴晶粒的聚集用于费-托的条件调查合成(FTS)反应。合成气进料中过量CO 2的存在极大地改变了催化活性和产物分布,特别是在CoAl催化剂上。在过量的CO 2下,失活速度相对较快,C 5+的选择性较低与CoSi相比,在CoAl上观察到环境,在相同的反应条件下也相当稳定。通过对钴微晶还原-氧化行为的测量,可以看出,CO 2分子通过部分氧化暴露的金属钴表面而起到了弱氧化剂的作用。与CoAl相比,CO 2环境下CO转化率的急剧下降和CH 4选择性的增加主要归因于金属钴表面通过与Al 2 O 3的强相互作用而无法还原的氧化支持。同时,在CoSi上的边际失活速率和较低的选择性变化主要归因于通过与SiO 2载体形成相对较弱的相互作用的较大的钴微晶,金属钴微晶具有可逆的氧化还原性质。在FTS催化剂上过度暴露于CO 2的弱氧化剂通常会通过形成强烈相互作用的大钴微晶而不可逆地降低催化活性,这在酸性Co / Al 2 O 3上比在Co / SiO 2催化剂上更常见。SiO 2上表面金属钴微晶的氧化还原的易逆性即使在过量的CO 2存在下,也可以防止FTS反应期间催化剂失活。
更新日期:2017-09-19
中文翻译:

CO 2对CO加氢制烃过程中Co / Al 2 O 3和Co / SiO 2失活行为的影响
原型的不同失活行为的Co /γ-Al系2 ö 3(煤)和Co /二氧化硅2过量CO下(硅化钴)的催化剂2环境中在表面氧化和钴晶粒的聚集用于费-托的条件调查合成(FTS)反应。合成气进料中过量CO 2的存在极大地改变了催化活性和产物分布,特别是在CoAl催化剂上。在过量的CO 2下,失活速度相对较快,C 5+的选择性较低与CoSi相比,在CoAl上观察到环境,在相同的反应条件下也相当稳定。通过对钴微晶还原-氧化行为的测量,可以看出,CO 2分子通过部分氧化暴露的金属钴表面而起到了弱氧化剂的作用。与CoAl相比,CO 2环境下CO转化率的急剧下降和CH 4选择性的增加主要归因于金属钴表面通过与Al 2 O 3的强相互作用而无法还原的氧化支持。同时,在CoSi上的边际失活速率和较低的选择性变化主要归因于通过与SiO 2载体形成相对较弱的相互作用的较大的钴微晶,金属钴微晶具有可逆的氧化还原性质。在FTS催化剂上过度暴露于CO 2的弱氧化剂通常会通过形成强烈相互作用的大钴微晶而不可逆地降低催化活性,这在酸性Co / Al 2 O 3上比在Co / SiO 2催化剂上更常见。SiO 2上表面金属钴微晶的氧化还原的易逆性即使在过量的CO 2存在下,也可以防止FTS反应期间催化剂失活。



























 京公网安备 11010802027423号
京公网安备 11010802027423号