当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expanding the Family of Hoveyda–Grubbs Catalysts Containing Unsymmetrical NHC Ligands
Organometallics ( IF 2.5 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.organomet.7b00488 Veronica Paradiso 1 , Valerio Bertolasi 2 , Chiara Costabile 1 , Tonino Caruso 1 , Michał Dąbrowski 3 , Karol Grela 3 , Fabia Grisi 1
Organometallics ( IF 2.5 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.organomet.7b00488 Veronica Paradiso 1 , Valerio Bertolasi 2 , Chiara Costabile 1 , Tonino Caruso 1 , Michał Dąbrowski 3 , Karol Grela 3 , Fabia Grisi 1
Affiliation
|
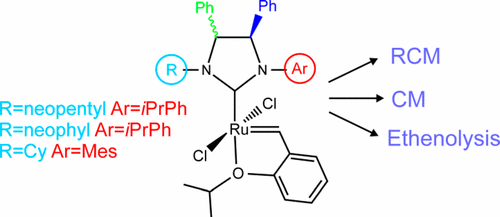
A series of Hoveyda–Grubbs second-generation catalysts containing N-alkyl/N′-aryl N-heterocyclic carbene (NHC) ligands were synthesized and investigated in representative olefin metathesis reactions. Steric perturbations of unsymmetrical NHCs were achieved through modulation of the hindrance of alkyl (neopentyl, neophyl, cyclohexyl) and aryl (2-isopropylphenyl, mesityl) substituents at the nitrogen atoms in combination with different backbone configurations (syn and anti). The NHC substitution patterns strongly influence the stability and reactivity of the corresponding complexes. In general, complexes bearing an anti NHC backbone are more stable and more active than their corresponding syn isomers. In both the series, the presence of bulky, highly branched N-alkyl groups tends to give reduced catalytic differences between syn and anti isomers, whereas the nature of the N′-aryl substituent (2-isopropylphenyl or mesityl) gives rise to different activity and/or selectivity. Of note, an N′-mesityl catalyst with anti backbone was found to be highly competent in the ethenolysis of ethyl oleate, achieving up to 90% selectivity for the formation of terminal olefins.
中文翻译:

扩大包含不对称NHC配体的Hoveyda-Grubbs催化剂的家族
合成了一系列含有N-烷基/ N'-芳基N-杂环卡宾(NHC)配体的Hoveyda-Grubbs第二代催化剂,并在代表性的烯烃复分解反应中进行了研究。通过调节氮原子上烷基(新戊基,新叶,环己基)和芳基(2-异丙基苯基,均三甲苯基)取代基与不同骨架结构(顺式和反式)结合的位阻,实现了不对称NHC的立体扰动。NHC取代模式强烈影响相应配合物的稳定性和反应性。通常,带有抗NHC主链的复合物比其相应的Syn更为稳定,活性更高。异构体。在这两个系列中,庞大的,高度支化的N-烷基基团的存在往往会降低顺式异构体和反式异构体之间的催化差异,而N'-芳基取代基(2-异丙基苯基或1,3,3,5-三甲苯基)的性质引起不同的活性。和/或选择性。值得注意的是,一个Ñ '与-基催化剂的抗骨干被发现是在油酸乙酯的乙烯醇分解高度胜任,实现高达90%的选择性为端烯烃的形成。
更新日期:2017-09-19
中文翻译:

扩大包含不对称NHC配体的Hoveyda-Grubbs催化剂的家族
合成了一系列含有N-烷基/ N'-芳基N-杂环卡宾(NHC)配体的Hoveyda-Grubbs第二代催化剂,并在代表性的烯烃复分解反应中进行了研究。通过调节氮原子上烷基(新戊基,新叶,环己基)和芳基(2-异丙基苯基,均三甲苯基)取代基与不同骨架结构(顺式和反式)结合的位阻,实现了不对称NHC的立体扰动。NHC取代模式强烈影响相应配合物的稳定性和反应性。通常,带有抗NHC主链的复合物比其相应的Syn更为稳定,活性更高。异构体。在这两个系列中,庞大的,高度支化的N-烷基基团的存在往往会降低顺式异构体和反式异构体之间的催化差异,而N'-芳基取代基(2-异丙基苯基或1,3,3,5-三甲苯基)的性质引起不同的活性。和/或选择性。值得注意的是,一个Ñ '与-基催化剂的抗骨干被发现是在油酸乙酯的乙烯醇分解高度胜任,实现高达90%的选择性为端烯烃的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号