当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Reactions of Aryl Tin(II) Hydrides {AriPr6Sn(μ-H)}2 (AriPr6 = C6H3-2,6-(C6H2-2,4,6-iPr3)2) and {AriPr4Sn(μ-H)}2 (AriPr4 = C6H3-2,6-(C6H3-2,6-iPr2)2) with Aryl Alkynes: Substituent Dependent Structural Isomers
Organometallics ( IF 2.5 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.organomet.7b00570 Madison L. McCrea-Hendrick 1 , Shuai Wang 1 , Kelly L. Gullett 1 , James C. Fettinger 1 , Philip P. Power 1
Organometallics ( IF 2.5 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.organomet.7b00570 Madison L. McCrea-Hendrick 1 , Shuai Wang 1 , Kelly L. Gullett 1 , James C. Fettinger 1 , Philip P. Power 1
Affiliation
|
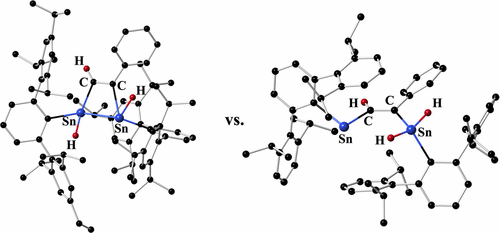
The reactions of the aryl tin(II) hydrides {AriPr6Sn(μ-H)}2 (AriPr6 = C6H3-2,6-(C6H2-2,4,6-iPr3)2) and {AriPr4Sn(μ-H)}2 (AriPr4 = C6H3-2,6-(C6H3-2,6-iPr2)2) with aryl alkynes were investigated. Reaction of {AriPr6Sn(μ-H)}2 and {AriPr4Sn(μ-H)}2 with 2 equiv of diphenyl acetylene, PhCCPh, afforded the aryl alkenyl stannylenes AriPr6SnC(Ph)C(H)Ph (1) and AriPr4SnC(Ph)C(H)Ph (2). In contrast, the analogous reactions of {AriPr6Sn(μ-H)}2 with 2 equiv of phenyl acetylene, HCCPh, afforded a high yield of the cis-1,2 addition product AriPr6(H) SnC(H)C(Ph)
SnC(H)C(Ph) Sn(H)AriPr6 (3), which has a four-membered Sn2C2 core structure comprised of two Sn–Sn bonded Sn(H)AriPr6 units bridged by a −C(H)═C(Ph)– moiety. The corresponding reaction of the less bulky hydride {AriPr4Sn(μ-H)}2 with 2 equiv of phenyl acetylene leads to AriPr4SnC(H)C(Ph)Sn(H)2AriPr4 (4) which unlike 3 has no Sn–Sn bonding. Instead, the tin atoms are connected solely by a −C(H)═C(Ph)– moiety. Each tin atom carries a AriPr4 substituent but one is also substituted by two hydrogens. The difference in behavior between PhCCPh and HCCPh is attributed mainly to the difference in steric bulk of the two substrates. The different products 3 and 4 are probably a consequence of the difference in size and dispersion force interactions of the AriPr6 and AriPr4 substituents. Compounds 1–4 were characterized by 1H, 13C, and 119Sn NMR, UV–vis, and IR spectroscopy and structurally by X-ray crystallography.
Sn(H)AriPr6 (3), which has a four-membered Sn2C2 core structure comprised of two Sn–Sn bonded Sn(H)AriPr6 units bridged by a −C(H)═C(Ph)– moiety. The corresponding reaction of the less bulky hydride {AriPr4Sn(μ-H)}2 with 2 equiv of phenyl acetylene leads to AriPr4SnC(H)C(Ph)Sn(H)2AriPr4 (4) which unlike 3 has no Sn–Sn bonding. Instead, the tin atoms are connected solely by a −C(H)═C(Ph)– moiety. Each tin atom carries a AriPr4 substituent but one is also substituted by two hydrogens. The difference in behavior between PhCCPh and HCCPh is attributed mainly to the difference in steric bulk of the two substrates. The different products 3 and 4 are probably a consequence of the difference in size and dispersion force interactions of the AriPr6 and AriPr4 substituents. Compounds 1–4 were characterized by 1H, 13C, and 119Sn NMR, UV–vis, and IR spectroscopy and structurally by X-ray crystallography.
中文翻译:

芳基锡(II)氢化物{Ar i Pr6 Sn(μ-H)} 2的反应(Ar i Pr6 = C 6 H 3 -2,6-(C 6 H 2 -2,4,6- i Pr 3)2)和{Ar i Pr4 Sn(μ-H)} 2(Ar i Pr4 = C 6 H 3 -2,6-(C 6 H 3 -2,6- i Pr 2)2)与芳基炔烃:取代基依赖性结构异构体
芳基锡(II)氢化物{Ar i Pr6 Sn(μ-H)} 2的反应(Ar i Pr6 = C 6 H 3 -2,6-(C 6 H 2 -2,4,6- i Pr 3)2)和{Ar i Pr4 Sn(μ-H)} 2(Ar i Pr4 = C 6 H 3 -2,6-(C 6 H 3 -2,6- i Pr 2)2)与芳基炔烃被调查了。{Ar i Pr6 Sn(μ-H)} 2的反应和具有2当量的二苯基乙炔PhCCPh的{Ar i Pr4 Sn(μ-H)} 2,得到芳基烯基亚锡基Ar i Pr6 SnC(Ph)C(H)Ph(1)和Ar i Pr4 SnC(Ph) C(H)Ph(2)。相反,{Ar i Pr6 Sn(μ-H)} 2与2当量的苯基乙炔HCCPh的类似反应提供了高收率的顺式-1,2加成产物Ar i Pr6(H) SnC(H具有四元Sn 2 C 2的)C(Ph)
SnC(H具有四元Sn 2 C 2的)C(Ph) Sn(H)Ar i Pr6(3)核心结构由两个通过-C(H)═C(Ph)-部分桥接的Sn-Sn键合的Sn(H)Ar i Pr6单元组成。较小体积的氢化物{Ar i Pr4 Sn(μ-H)} 2与2当量的苯基乙炔的相应反应导致Ar i Pr4 SnC(H)C(Ph)Sn(H)2 Ar i Pr4(4)与3不同的是,它没有Sn-Sn键。相反,锡原子仅通过-C(H)═C(Ph)–部分连接。每个锡原子带有一个Ar i Pr4取代基,但一个也被两个氢取代。PhCCPh和HCCPh之间行为的差异主要归因于两种底物的空间体积差异。不同的产物3和4可能是Ar i Pr6和Ar i Pr4取代基的大小和分散力相互作用不同的结果。化合物1 - 4由进行了表征1个H,13 C,和119的Sn NMR,UV-VIS,和IR光谱和结构上由X射线晶体学。
Sn(H)Ar i Pr6(3)核心结构由两个通过-C(H)═C(Ph)-部分桥接的Sn-Sn键合的Sn(H)Ar i Pr6单元组成。较小体积的氢化物{Ar i Pr4 Sn(μ-H)} 2与2当量的苯基乙炔的相应反应导致Ar i Pr4 SnC(H)C(Ph)Sn(H)2 Ar i Pr4(4)与3不同的是,它没有Sn-Sn键。相反,锡原子仅通过-C(H)═C(Ph)–部分连接。每个锡原子带有一个Ar i Pr4取代基,但一个也被两个氢取代。PhCCPh和HCCPh之间行为的差异主要归因于两种底物的空间体积差异。不同的产物3和4可能是Ar i Pr6和Ar i Pr4取代基的大小和分散力相互作用不同的结果。化合物1 - 4由进行了表征1个H,13 C,和119的Sn NMR,UV-VIS,和IR光谱和结构上由X射线晶体学。
更新日期:2017-09-19
 SnC(H)C(Ph)
SnC(H)C(Ph) Sn(H)AriPr6 (3), which has a four-membered Sn2C2 core structure comprised of two Sn–Sn bonded Sn(H)AriPr6 units bridged by a −C(H)═C(Ph)– moiety. The corresponding reaction of the less bulky hydride {AriPr4Sn(μ-H)}2 with 2 equiv of phenyl acetylene leads to AriPr4SnC(H)C(Ph)Sn(H)2AriPr4 (4) which unlike 3 has no Sn–Sn bonding. Instead, the tin atoms are connected solely by a −C(H)═C(Ph)– moiety. Each tin atom carries a AriPr4 substituent but one is also substituted by two hydrogens. The difference in behavior between PhCCPh and HCCPh is attributed mainly to the difference in steric bulk of the two substrates. The different products 3 and 4 are probably a consequence of the difference in size and dispersion force interactions of the AriPr6 and AriPr4 substituents. Compounds 1–4 were characterized by 1H, 13C, and 119Sn NMR, UV–vis, and IR spectroscopy and structurally by X-ray crystallography.
Sn(H)AriPr6 (3), which has a four-membered Sn2C2 core structure comprised of two Sn–Sn bonded Sn(H)AriPr6 units bridged by a −C(H)═C(Ph)– moiety. The corresponding reaction of the less bulky hydride {AriPr4Sn(μ-H)}2 with 2 equiv of phenyl acetylene leads to AriPr4SnC(H)C(Ph)Sn(H)2AriPr4 (4) which unlike 3 has no Sn–Sn bonding. Instead, the tin atoms are connected solely by a −C(H)═C(Ph)– moiety. Each tin atom carries a AriPr4 substituent but one is also substituted by two hydrogens. The difference in behavior between PhCCPh and HCCPh is attributed mainly to the difference in steric bulk of the two substrates. The different products 3 and 4 are probably a consequence of the difference in size and dispersion force interactions of the AriPr6 and AriPr4 substituents. Compounds 1–4 were characterized by 1H, 13C, and 119Sn NMR, UV–vis, and IR spectroscopy and structurally by X-ray crystallography.
中文翻译:

芳基锡(II)氢化物{Ar i Pr6 Sn(μ-H)} 2的反应(Ar i Pr6 = C 6 H 3 -2,6-(C 6 H 2 -2,4,6- i Pr 3)2)和{Ar i Pr4 Sn(μ-H)} 2(Ar i Pr4 = C 6 H 3 -2,6-(C 6 H 3 -2,6- i Pr 2)2)与芳基炔烃:取代基依赖性结构异构体
芳基锡(II)氢化物{Ar i Pr6 Sn(μ-H)} 2的反应(Ar i Pr6 = C 6 H 3 -2,6-(C 6 H 2 -2,4,6- i Pr 3)2)和{Ar i Pr4 Sn(μ-H)} 2(Ar i Pr4 = C 6 H 3 -2,6-(C 6 H 3 -2,6- i Pr 2)2)与芳基炔烃被调查了。{Ar i Pr6 Sn(μ-H)} 2的反应和具有2当量的二苯基乙炔PhCCPh的{Ar i Pr4 Sn(μ-H)} 2,得到芳基烯基亚锡基Ar i Pr6 SnC(Ph)C(H)Ph(1)和Ar i Pr4 SnC(Ph) C(H)Ph(2)。相反,{Ar i Pr6 Sn(μ-H)} 2与2当量的苯基乙炔HCCPh的类似反应提供了高收率的顺式-1,2加成产物Ar i Pr6(H)
 SnC(H具有四元Sn 2 C 2的)C(Ph)
SnC(H具有四元Sn 2 C 2的)C(Ph) Sn(H)Ar i Pr6(3)核心结构由两个通过-C(H)═C(Ph)-部分桥接的Sn-Sn键合的Sn(H)Ar i Pr6单元组成。较小体积的氢化物{Ar i Pr4 Sn(μ-H)} 2与2当量的苯基乙炔的相应反应导致Ar i Pr4 SnC(H)C(Ph)Sn(H)2 Ar i Pr4(4)与3不同的是,它没有Sn-Sn键。相反,锡原子仅通过-C(H)═C(Ph)–部分连接。每个锡原子带有一个Ar i Pr4取代基,但一个也被两个氢取代。PhCCPh和HCCPh之间行为的差异主要归因于两种底物的空间体积差异。不同的产物3和4可能是Ar i Pr6和Ar i Pr4取代基的大小和分散力相互作用不同的结果。化合物1 - 4由进行了表征1个H,13 C,和119的Sn NMR,UV-VIS,和IR光谱和结构上由X射线晶体学。
Sn(H)Ar i Pr6(3)核心结构由两个通过-C(H)═C(Ph)-部分桥接的Sn-Sn键合的Sn(H)Ar i Pr6单元组成。较小体积的氢化物{Ar i Pr4 Sn(μ-H)} 2与2当量的苯基乙炔的相应反应导致Ar i Pr4 SnC(H)C(Ph)Sn(H)2 Ar i Pr4(4)与3不同的是,它没有Sn-Sn键。相反,锡原子仅通过-C(H)═C(Ph)–部分连接。每个锡原子带有一个Ar i Pr4取代基,但一个也被两个氢取代。PhCCPh和HCCPh之间行为的差异主要归因于两种底物的空间体积差异。不同的产物3和4可能是Ar i Pr6和Ar i Pr4取代基的大小和分散力相互作用不同的结果。化合物1 - 4由进行了表征1个H,13 C,和119的Sn NMR,UV-VIS,和IR光谱和结构上由X射线晶体学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号