当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydration Structure of Brookite TiO2 (210)
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.jpcc.7b05524 Eero Holmström 1 , Simiam Ghan 1 , Hitoshi Asakawa , Yasuhiro Fujita , Takeshi Fukuma 2 , Sunao Kamimura 3 , Teruhisa Ohno 2, 3 , Adam S. Foster 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.jpcc.7b05524 Eero Holmström 1 , Simiam Ghan 1 , Hitoshi Asakawa , Yasuhiro Fujita , Takeshi Fukuma 2 , Sunao Kamimura 3 , Teruhisa Ohno 2, 3 , Adam S. Foster 1
Affiliation
|
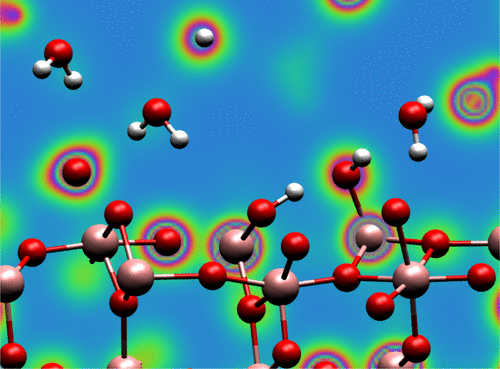
The interface of TiO2 and water has been heavily researched due to the photocatalytical capabilities of this system. Whereas the majority of existing work has targeted the rutile and anatase phases of TiO2, much less is known about the brookite phase. In this work, we use first-principles molecular dynamics simulations to find the hydration structure of the brookite (210) surface. We find both pure water and an aqueous solution of KCl to order laterally at the sites of surface Ti cations due to electrostatic and chemical considerations, qualitatively in agreement with experimental high-resolution atomic force microscopy measurements. A significant fraction of surface oxygens is hydroxylated for all cases, with up to 40% realized for the aqueous solution at bulk coverage, a result originating in orientational constraints placed on water near the solvated K and Cl ions. Proton transfer is nearly equally frequent between the surface and liquid regions and within the liquid region, but the presence of K and Cl ions makes proton transfer less efficient.
中文翻译:

板钛矿TiO 2(210)的水合结构
由于该系统的光催化能力,已经对TiO 2和水的界面进行了大量研究。鉴于大多数现有工作的目标是TiO 2的金红石相和锐钛矿相,关于板钛矿相的了解还很少。在这项工作中,我们使用第一性原理分子动力学模拟来发现板钛矿(210)表面的水合结构。由于静电和化学方面的考虑,我们发现纯水和KCl水溶液在表面Ti阳离子的位置上横向排列,定性地与实验高分辨率原子力显微镜测量相吻合。在所有情况下,大部分表面氧都被羟基化,在大面积覆盖时,水溶液达到40%,这是由于溶剂化的K和Cl离子附近的水受到取向限制而导致的。在表面区域和液体区域之间以及在液体区域内,质子转移几乎同样频繁,但是K和Cl离子的存在会使质子转移效率降低。
更新日期:2017-09-19
中文翻译:

板钛矿TiO 2(210)的水合结构
由于该系统的光催化能力,已经对TiO 2和水的界面进行了大量研究。鉴于大多数现有工作的目标是TiO 2的金红石相和锐钛矿相,关于板钛矿相的了解还很少。在这项工作中,我们使用第一性原理分子动力学模拟来发现板钛矿(210)表面的水合结构。由于静电和化学方面的考虑,我们发现纯水和KCl水溶液在表面Ti阳离子的位置上横向排列,定性地与实验高分辨率原子力显微镜测量相吻合。在所有情况下,大部分表面氧都被羟基化,在大面积覆盖时,水溶液达到40%,这是由于溶剂化的K和Cl离子附近的水受到取向限制而导致的。在表面区域和液体区域之间以及在液体区域内,质子转移几乎同样频繁,但是K和Cl离子的存在会使质子转移效率降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号