当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Synthesis of Alkynylated Isoquinolines and Biisoquinolines via RhIII Catalyzed C–H Activation/1,3-Diyne Strategy
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.joc.7b01867 Ruokun Feng 1 , Hanqi Ning 1 , Han Su 1 , Yuan Gao 1 , Haotian Yin 1 , Yudan Wang 1 , Zhen Yang 1 , Chenze Qi 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.joc.7b01867 Ruokun Feng 1 , Hanqi Ning 1 , Han Su 1 , Yuan Gao 1 , Haotian Yin 1 , Yudan Wang 1 , Zhen Yang 1 , Chenze Qi 1
Affiliation
|
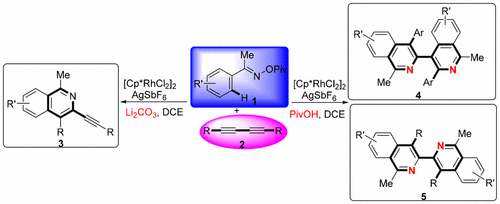
Described herein is a convenient and highly selective synthesis of alkynylated isoquinolines and biisoquinolines from various aryl ketone O-pivaloyloxime derivatives and 1,3-diynes via rhodium-catalyzed C–H bond activation. In this transformations, alkynylated isoquinolines, 3,4′- and 3,3′-biisoquinolines could be obtained respectively through changing the reaction conditions. Mechanistic investigation revealed that the C–H activation of aryl ketone O-pivaloyloxime was the key step to this reaction.
中文翻译:

Rh III催化的C–H活化/ 1,3-二炔策略选择性合成炔基化异喹啉和双异喹啉
本文描述的是通过铑催化的C–H键活化,由各种芳基酮O-新戊酰肟衍生物和1,3-二炔方便而高度选择性地合成炔基化异喹啉和双异喹啉。在该转化中,通过改变反应条件,可以分别获得炔基化的异喹啉,3,4'-和3,3'-二异喹啉。机理研究表明,芳基酮O-新戊酰肟的C–H活化是该反应的关键步骤。
更新日期:2017-09-19
中文翻译:

Rh III催化的C–H活化/ 1,3-二炔策略选择性合成炔基化异喹啉和双异喹啉
本文描述的是通过铑催化的C–H键活化,由各种芳基酮O-新戊酰肟衍生物和1,3-二炔方便而高度选择性地合成炔基化异喹啉和双异喹啉。在该转化中,通过改变反应条件,可以分别获得炔基化的异喹啉,3,4'-和3,3'-二异喹啉。机理研究表明,芳基酮O-新戊酰肟的C–H活化是该反应的关键步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号