当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Near-Infrared Photoswitching of Azobenzenes under Physiological Conditions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/jacs.7b06471 Mingxin Dong 1, 2 , Amirhossein Babalhavaeji 1 , Catherine V. Collins 1 , Kareem Jarrah 1 , Oleg Sadovski 1 , Qiuyun Dai 2 , G. Andrew Woolley 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/jacs.7b06471 Mingxin Dong 1, 2 , Amirhossein Babalhavaeji 1 , Catherine V. Collins 1 , Kareem Jarrah 1 , Oleg Sadovski 1 , Qiuyun Dai 2 , G. Andrew Woolley 1
Affiliation
|
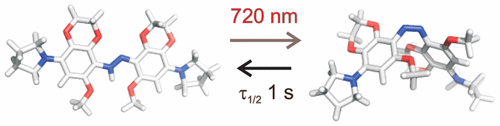
Biological tissue exhibits an absorbance minimum in the near-infrared between 700 and 900 nm that permits deep penetration of light. Molecules that undergo photoisomerization in this bio-optical window are highly desirable as core structures for the development of photopharmaceuticals and as components of chemical-biological tools. We report the systematic design, synthesis, and testing of an azobenzene derivative tailored to undergo single-photon photoswitching with near-infrared light under physiological conditions. A fused dioxane ring and a methoxy substituent were used to place oxygen atoms in all four ortho positions, as well as two meta positions, relative to the azobenzene N═N double bond. This substitution pattern, together with a para pyrrolidine group, raises the pKa of the molecule so that it is protonated at physiological pH and absorbs at wavelengths >700 nm. This azobenzene derivative, termed DOM-azo, is stable for months in neutral aqueous solutions, undergoes trans-to-cis photoswitching with 720 nm light, and thermally reverts to the stable trans isomer with a half-life near 1 s.
中文翻译:

生理条件下偶氮苯的近红外光开关
生物组织在700到900 nm之间的近红外光中显示出最小的吸光度,可以使光线深入穿透。在该生物光学窗口中经历光异构化的分子作为光药物开发的核心结构和化学生物学工具的组成是非常理想的。我们报告了系统设计,合成和测试的偶氮苯衍生物量身定制的单光子光开关与近红外光在生理条件下。相对于偶氮苯N = N双键,使用稠合的二恶烷环和甲氧基取代基将氧原子置于所有四个邻位以及两个间位。此取代模式,有一起对吡咯烷基团提高了分子的p K a,使其在生理pH下被质子化,并在> 700 nm的波长处吸收。此偶氮苯衍生物被称为DOM偶氮,是用于在中性水溶液个月是稳定,进行反式-到-顺式与720nm的光控光,并热回复到稳定的反式与接近1秒的半衰期异构体。
更新日期:2017-09-19
中文翻译:

生理条件下偶氮苯的近红外光开关
生物组织在700到900 nm之间的近红外光中显示出最小的吸光度,可以使光线深入穿透。在该生物光学窗口中经历光异构化的分子作为光药物开发的核心结构和化学生物学工具的组成是非常理想的。我们报告了系统设计,合成和测试的偶氮苯衍生物量身定制的单光子光开关与近红外光在生理条件下。相对于偶氮苯N = N双键,使用稠合的二恶烷环和甲氧基取代基将氧原子置于所有四个邻位以及两个间位。此取代模式,有一起对吡咯烷基团提高了分子的p K a,使其在生理pH下被质子化,并在> 700 nm的波长处吸收。此偶氮苯衍生物被称为DOM偶氮,是用于在中性水溶液个月是稳定,进行反式-到-顺式与720nm的光控光,并热回复到稳定的反式与接近1秒的半衰期异构体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号