当前位置:
X-MOL 学术
›
Mater. Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The stability and transformation of TiC with different stoichiometries in Cu–Si melts
Materials & Design ( IF 7.6 ) Pub Date : 2017-12-01 , DOI: 10.1016/j.matdes.2017.09.030 Haimin Ding , Xianlong Wang , Qing Liu , Jinfeng Wang , Chunyan Li , Xinchun Zhang
Materials & Design ( IF 7.6 ) Pub Date : 2017-12-01 , DOI: 10.1016/j.matdes.2017.09.030 Haimin Ding , Xianlong Wang , Qing Liu , Jinfeng Wang , Chunyan Li , Xinchun Zhang
|
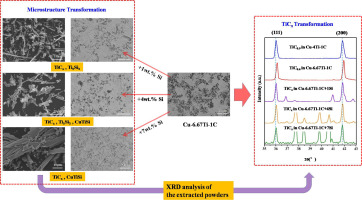
Abstract In this work, the stability and transformation of two kinds of TiC with different stoichiometries in Cu–Si melts are studied, respectively. It is found that the near-stoichiometirc TiC with fewer carbon vacancies (designated as TiCx), such as TiC0.9, is relatively stable in Cu–Si melts, while the non-stoichiometric TiC with more carbon vacancies (designated as TiCy), such as TiC0.6, is unstable. And TiCy tends to transform into TiCx. Meanwhile, some Ti element from TiCy will be dissolved into the melts. It is considered that the addition of Si influences the equilibrium between TiC with different stoichiometries and soluble Ti in Cu melts, which results in the transformation of TiC. According to the results, the stoichiometry of TiC must be controlled when it is used as the reinforced phase in Cu–Si alloys.
中文翻译:

不同化学计量的 TiC 在 Cu-Si 熔体中的稳定性和转变
摘要 本文分别研究了两种不同化学计量比的 TiC 在 Cu-Si 熔体中的稳定性和相变。发现具有较少碳空位(指定为 TiCx)的接近化学计量的 TiC,例如 TiC0.9,在 Cu-Si 熔体中相对稳定,而具有更多碳空位的非化学计量的 TiC(指定为 TiCy),如TiC0.6,是不稳定的。并且 TiCy 倾向于转化为 TiCx。同时,TiCy 中的一些 Ti 元素会溶解到熔体中。认为Si的加入影响了不同化学计量的TiC与Cu熔体中可溶性Ti之间的平衡,从而导致了TiC的转变。根据结果,当 TiC 用作 Cu-Si 合金中的增强相时,必须控制其化学计量。
更新日期:2017-12-01
中文翻译:

不同化学计量的 TiC 在 Cu-Si 熔体中的稳定性和转变
摘要 本文分别研究了两种不同化学计量比的 TiC 在 Cu-Si 熔体中的稳定性和相变。发现具有较少碳空位(指定为 TiCx)的接近化学计量的 TiC,例如 TiC0.9,在 Cu-Si 熔体中相对稳定,而具有更多碳空位的非化学计量的 TiC(指定为 TiCy),如TiC0.6,是不稳定的。并且 TiCy 倾向于转化为 TiCx。同时,TiCy 中的一些 Ti 元素会溶解到熔体中。认为Si的加入影响了不同化学计量的TiC与Cu熔体中可溶性Ti之间的平衡,从而导致了TiC的转变。根据结果,当 TiC 用作 Cu-Si 合金中的增强相时,必须控制其化学计量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号