Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.bmc.2017.09.009 Pervaiz Ali Channar , Aamer Saeed , Fayaz Ali Larik , Muhammad Rafiq , Zaman Ashraf , Farukh Jabeen , Tanzeela Abdul Fattah
|
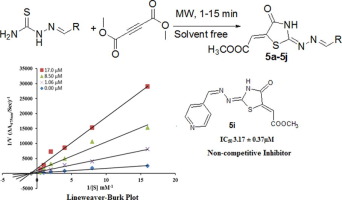
The present article describes the synthesis and enzyme inhibitory kinetics of methyl[2-(arylmethylene-hydrazono)-4-oxo-thiazolidin-5-ylidene]acetates 5a–j as mushroom tyrosinase inhibitors. The title compounds were synthesized via cyclocondensation of thiosemicarbazones 3a–j with dimethyl but-2-ynedioate (DMAD) 4 in good yields under solvent-free conditions. The synthesized compounds were evaluated for their potential to inhibit the activity of mushroom tyrosinase. It was unveiled that compounds 5i showed excellent enzyme inhibitory activity with IC50 3.17 µM while IC50 of standard kojic acid is 15.91 µM. The presence of heterocyclic pyridine ring in compound 5i play important role in enzyme inhibitory activity as rest of the functional groups are common in all synthesized compounds. The enzyme inhibitory kinetics of the most potent derivative 5i determined by Lineweaver-Burk plots and Dixon plots showed that it is non-competitive inhibitor with Ki value 1.5 µM. It was further investigated that the wet lab results are in good agreement with the computational results. The molecular docking of the synthesized compounds was performed against tyrosinase protein (PDBID 2Y9X) to delineate ligand-protein interactions at molecular level. The docking results showed that the major interacting residues are His244, His85, His263, Val 283, His 296, Asn260, Val248, His260, His261 and Phe264 which are located in active binding site of the protein. The molecular modeling demonstrates that the oxygen atom of the compound 5i coordinated with the key residues in the active site of mushroom tyrosinase contribute significantly against inhibitory ability and diminishing the human melanin synthesis. These results evident that compound 5i is a lead structure in developing most potent mushroom tyrosinase inhibitors.
中文翻译:

蘑菇酪氨酸酶抑制剂的取代甲基[2-(4-二甲基氨基-亚苄基)-肼基)-4-氧代-噻唑烷--5-亚甲基]乙酸酯的合成,计算研究和酶抑制动力学
本文描述了作为蘑菇酪氨酸酶抑制剂的甲基[2-(芳基亚甲基-肼基)-4-氧代-噻唑烷-5--5-亚甲基]乙酸酯5a – j的合成和酶抑制动力学。标题化合物是在无溶剂条件下,以高收率通过硫代半咔唑3a – j与丁-2-炔二酸二甲酯(DMAD)4的环缩合反应合成的。评价合成的化合物抑制蘑菇酪氨酸酶活性的潜力。据透露,化合物5i在IC 50为3.17 µM时显示出优异的酶抑制活性,而IC 50为标准曲酸的浓度为15.91 µM。化合物5i中杂环吡啶环的存在在酶抑制活性中起重要作用,因为所有合成的化合物中其余的官能团都是常见的。通过Lineweaver-Burk图和Dixon图确定的最有效衍生物5i的酶抑制动力学表明,它是与Ki不竞争的抑制剂值1.5 µM。进一步研究表明,湿实验室的结果与计算结果非常吻合。对酪氨酸酶蛋白(PDBID 2Y9X)进行合成化合物的分子对接,以在分子水平上描述配体-蛋白相互作用。对接结果表明主要的相互作用残基是His244,His85,His263,Val 283,His 296,Asn260,Val248,His260,His261和Phe264,它们位于蛋白的活性结合位点。分子模型表明,化合物5i的氧原子与蘑菇酪氨酸酶活性位点中的关键残基配位,显着促进了其抑制能力并减少了人类黑色素的合成。这些结果表明,化合物5i 是开发最有效的蘑菇酪氨酸酶抑制剂的先导结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号