Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.tetlet.2017.09.040 Hemchandra K. Chaudhari , Akshata Pahelkar , Balaram S. Takale
|
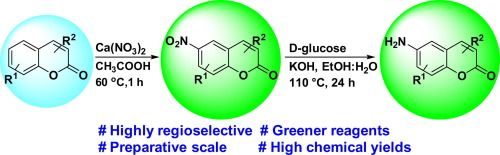
In contrast to the conventional deleterious approach for nitration (for example HNO3/H2SO4) and for reduction (for example Zn/HCl), we hypothesized that sensitive heterocycles such as coumarins could not withstand with those hard conditions. Hence, while studying this reaction sequence to prepare amino coumarins (which is our ongoing project to synthesize antitubercular coumarin agents), we came across mild and greener reagent for nitration using calcium nitrate (Ca(NO3)2·4H2O; lime nitrate), and reduction using d-glucose. These two mild, chemoselective, high yielding methods are discussed herein.
中文翻译:

通过新的顺序硝化和还原方案进行氨基香豆素的制备规模合成
与传统的用于硝化(例如HNO 3 / H 2 SO 4)和用于还原(例如Zn / HCl)的有害方法相反,我们假设敏感的杂环(如香豆素)无法在那些困难的条件下承受。因此,在研究此反应顺序以制备氨基香豆素(这是我们正在进行的合成抗结核香豆素试剂的正在进行的项目)时,我们遇到了使用硝酸钙(Ca(NO 3)2 ·4H 2 O;硝酸钙)进行硝化的温和绿色试剂。),并使用d-葡萄糖还原。本文讨论了这两种温和的,化学选择性的,高产率的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号