当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioinspired chemical synthesis of monomeric and dimeric stephacidin A congeners
Nature Chemistry ( IF 19.2 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1038/nchem.2862 Ken Mukai , Danilo Pereira de Sant'Ana , Yasuo Hirooka , Eduardo V. Mercado-Marin , David E. Stephens , Kevin G. M. Kou , Sven C. Richter , Naomi Kelley , Richmond Sarpong
Nature Chemistry ( IF 19.2 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1038/nchem.2862 Ken Mukai , Danilo Pereira de Sant'Ana , Yasuo Hirooka , Eduardo V. Mercado-Marin , David E. Stephens , Kevin G. M. Kou , Sven C. Richter , Naomi Kelley , Richmond Sarpong
|
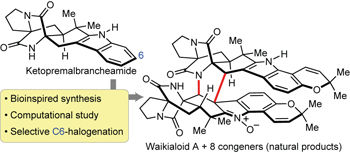
Stephacidin A and its congeners are a collection of secondary metabolites that possess intriguing structural motifs. They stem from unusual biosynthetic sequences that lead to the incorporation of a prenyl or reverse-prenyl group into a bicyclo[2.2.2]diazaoctane framework, a chromene unit or the vestige thereof. To complement biosynthetic studies, which normally play a significant role in unveiling the biosynthetic pathways of natural products, here we demonstrate that chemical synthesis can provide important insights into biosynthesis. We identify a short total synthesis of congeners in the reverse-prenylated indole alkaloid family related to stephacidin A by taking advantage of a direct indole C6 halogenation of the related ketopremalbrancheamide. This novel strategic approach has now made possible the syntheses of several natural products, including malbrancheamides B and C, notoamides F, I and R, aspergamide B, and waikialoid A, which is a heterodimer of avrainvillamide and aspergamide B. Our approach to the preparation of these prenylated and reverse-prenylated indole alkaloids is bioinspired, and may also inform the as-yet undetermined biosynthesis of several congeners.
中文翻译:

生物启发化学合成单体和二聚步酸A同类物
Stephacidin A及其同源物是具有有趣结构基序的次级代谢产物的集合。它们源自不寻常的生物合成序列,其导致异戊二烯基或反向异戊烯基基团掺入双环[2.2.2]二氮杂辛烷骨架,色烯单元或其残余物中。为了补充通常在揭示天然产物的生物合成途径中起重要作用的生物合成研究,在这里我们证明化学合成可以提供有关生物合成的重要见解。我们通过利用相关酮戊二烯丙基苯甲酰胺的直接吲哚C6卤化,确定了与Stephacidin A相关的反向异戊二烯基吲哚生物碱家族中同类物的短总合成。现在,这种新颖的战略方法使几种天然产物的合成成为可能,
更新日期:2017-09-20
中文翻译:

生物启发化学合成单体和二聚步酸A同类物
Stephacidin A及其同源物是具有有趣结构基序的次级代谢产物的集合。它们源自不寻常的生物合成序列,其导致异戊二烯基或反向异戊烯基基团掺入双环[2.2.2]二氮杂辛烷骨架,色烯单元或其残余物中。为了补充通常在揭示天然产物的生物合成途径中起重要作用的生物合成研究,在这里我们证明化学合成可以提供有关生物合成的重要见解。我们通过利用相关酮戊二烯丙基苯甲酰胺的直接吲哚C6卤化,确定了与Stephacidin A相关的反向异戊二烯基吲哚生物碱家族中同类物的短总合成。现在,这种新颖的战略方法使几种天然产物的合成成为可能,











































 京公网安备 11010802027423号
京公网安备 11010802027423号