当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective synthesis of sterically hindered α-allyl–α-aryl oxindoles via palladium-catalysed decarboxylative asymmetric allylic alkylation
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1039/c7ob02161e Mark Jackson 1, 2, 3, 4, 5 , Calvin Quince O'Broin 1, 2, 3, 4, 5 , Helge Müller-Bunz 1, 2, 3, 4, 5 , Patrick J. Guiry 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1039/c7ob02161e Mark Jackson 1, 2, 3, 4, 5 , Calvin Quince O'Broin 1, 2, 3, 4, 5 , Helge Müller-Bunz 1, 2, 3, 4, 5 , Patrick J. Guiry 1, 2, 3, 4, 5
Affiliation
|
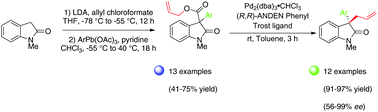
The highly enantioselective synthesis of sterically hindered α-allyl–α-aryl oxindoles possessing an all-carbon quaternary stereocenter at the oxindole 3-position has been developed. The key step in the synthetic route employed was a novel one-pot, two-step synthesis of α-aryl–β-amido allyl ester substituted oxindoles in good yields of 41–75% (13 examples) by interception of an unstable allyl ester intermediate through reaction with aryllead triacetate reagents. Pd-Catalyzed decarboxylative asymmetric allylic alkylation (DAAA) was optimized with 2,4,6-trimethoxyphenyl as the aryl-containing substrate. A screen of chiral P,N- and P,P-based ligands showed that the ANDEN-phenyl Trost ligand was the most effective, affording the corresponding α-allyl–α-aryl oxindole product in 96% yield and 99% ee. A substrate scope of a further 12 α-aryl–β-amido allyl ester substituted oxindoles showed that products containing bulky di-ortho-methoxy substituted arenes and naphthyl groups were formed in very high ee's (94–98%), whereas those lacking this substitution pattern were formed in more moderate levels of enantioselectivities (56–63% ee). Surprisingly, the 2,6-dimethylphenyl-substituted substrate afforded the O-allylated product in contrast to the expected C-allylated product. A crystal structure was obtained of the 2,4,6-trimethoxyphenyl-substituted α-allyl–α-aryl oxindole product which enabled us to identify the absolute stereochemistry of the quaternary stereocenter formed. A plausible explanation to rationalise the sense of enantioselection observed in these DAAA transformations is also proposed.
中文翻译:

通过钯催化的脱羧不对称烯丙基烷基化反应,对位合成α-烯丙基-α-芳基羟吲哚的对映选择性
已开发出在对羟基3位具有全碳四级立体中心的位阻α-烯丙基-α-芳基羟基吲哚的高对映选择性合成。合成途径中的关键步骤是新颖的一锅,两步合成α-芳基-β-酰胺基烯丙基酯取代的羟吲哚,通过拦截不稳定的烯丙基酯以41-75%的高收率(13个实例)通过与三乙酸芳基铅试剂反应生成中间体。以2,4,6-三甲氧基苯基作为含芳基的底物优化了钯催化的脱羧不对称烯丙基烷基化反应(DAAA)。手性P,N-和P,P的屏幕基配体表明,ANDEN-苯基Trost配体最有效,以96%的收率和99%ee的产率提供相应的α-烯丙基-α-芳基羟吲哚产物。另有12个α-芳基-β-酰胺基烯丙基酯取代的羟吲哚的底物范围显示,在高ee(94-98%)的情况下会形成含有庞大的二-邻甲氧基取代的芳烃和萘基的产物,而缺少这些的替代模式是在中等水平的对映选择性(56-63%ee)下形成的。出乎意料的是,与预期的C相反,2,6-二甲基苯基取代的底物提供了O-烯丙基化的产物烯丙基化的产品。获得了2,4,6-三甲氧基苯基取代的α-烯丙基-α-芳基羟吲哚产物的晶体结构,这使我们能够确定所形成的四级立体中心的绝对立体化学。还提出了合理的解释,以合理化在这些DAAA转换中观察到的对映异构感。
更新日期:2017-09-18
中文翻译:

通过钯催化的脱羧不对称烯丙基烷基化反应,对位合成α-烯丙基-α-芳基羟吲哚的对映选择性
已开发出在对羟基3位具有全碳四级立体中心的位阻α-烯丙基-α-芳基羟基吲哚的高对映选择性合成。合成途径中的关键步骤是新颖的一锅,两步合成α-芳基-β-酰胺基烯丙基酯取代的羟吲哚,通过拦截不稳定的烯丙基酯以41-75%的高收率(13个实例)通过与三乙酸芳基铅试剂反应生成中间体。以2,4,6-三甲氧基苯基作为含芳基的底物优化了钯催化的脱羧不对称烯丙基烷基化反应(DAAA)。手性P,N-和P,P的屏幕基配体表明,ANDEN-苯基Trost配体最有效,以96%的收率和99%ee的产率提供相应的α-烯丙基-α-芳基羟吲哚产物。另有12个α-芳基-β-酰胺基烯丙基酯取代的羟吲哚的底物范围显示,在高ee(94-98%)的情况下会形成含有庞大的二-邻甲氧基取代的芳烃和萘基的产物,而缺少这些的替代模式是在中等水平的对映选择性(56-63%ee)下形成的。出乎意料的是,与预期的C相反,2,6-二甲基苯基取代的底物提供了O-烯丙基化的产物烯丙基化的产品。获得了2,4,6-三甲氧基苯基取代的α-烯丙基-α-芳基羟吲哚产物的晶体结构,这使我们能够确定所形成的四级立体中心的绝对立体化学。还提出了合理的解释,以合理化在这些DAAA转换中观察到的对映异构感。



























 京公网安备 11010802027423号
京公网安备 11010802027423号