当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controllable assembly of Cu(II) coordination compounds based on a flexible zwitterionic benzimidazole–dicarboxylate ligand: synthesis, structural diversity, reversible SCSC transformation and magnetic properties
CrystEngComm ( IF 2.6 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1039/c7ce01215b Yan-Fang Feng 1, 2, 3, 4 , Qi Tang 1, 2, 3, 4 , Kai-Liang Luo 1, 2, 3, 4 , Ji-Qing Wu 1, 2, 3, 4 , Zhong Zhang 1, 2, 3, 4 , Yu-Ning Liang 1, 2, 3, 4 , Fu-Pei Liang 1, 2, 3, 4
CrystEngComm ( IF 2.6 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1039/c7ce01215b Yan-Fang Feng 1, 2, 3, 4 , Qi Tang 1, 2, 3, 4 , Kai-Liang Luo 1, 2, 3, 4 , Ji-Qing Wu 1, 2, 3, 4 , Zhong Zhang 1, 2, 3, 4 , Yu-Ning Liang 1, 2, 3, 4 , Fu-Pei Liang 1, 2, 3, 4
Affiliation
|
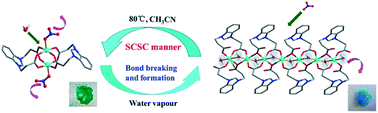
A systematic investigation on the assembly of a flexible zwitterionic ligand, 1,3-bis(2-carboxyethyl)benzimidazolium (HL), with various Cu(II) salts under different conditions afforded nine coordination compounds: [Cu2L2(NO3)2]·CH3CN (1), [Cu2L2(H2O)2](NO3)2·5H2O (2), {[CuL(H2O)](NO3)}n (3), [Cu4L4(OH)2(H2O)2](NO3)2·10H2O (4), [Cu2L2(H2O)2](ClO4)2 (5), [CuL(H2O)3]2SO4·7H2O (6), [Cu(HL)2Cl2] (7), [Cu2L2Cl2]·2H2O (8) and {[Cu2L2Cl2]·2H2O}n (9). Compounds 1 and 8 are neutral paddle-wheel shaped dinuclear clusters with either nitrate or chloride as the axial ligand, while 2 and 5 are composed of identical [Cu2L2(H2O)2]2+ cationic units displaying a paddle-wheel motif with an axially coordinated aqua ligand but different counter anions. Compound 3 exhibits a 1D linear structure, where two adjacent Cu(II) centers are triply bridged via two carboxylate groups plus one aqua ligand. Compound 4 is a tetranuclear cluster supported by two μ3-bridged OH− groups and four carboxylate bridges. Compounds 6 and 9 are mononuclear species constructed from the dicarboxylate ligand in two different coordination modes. Compound 9 assumes a 1D looped chain topology with paddle-wheel cluster nodes propagated by pairs of flexible linkers. A close examination revealed that the conformational freedom of the flexible dicarboxylate ligand as well as the labile binding modes of its two side arm carboxylates is sensitive to the reaction conditions, thus resulted in the different structural types of these coordination compounds. The solvent-induced reversible single crystal-to-single crystal (SCSC) transformation between 1 and 3 could be easily achieved via the shuttle movement of NO3− outward and inward of the Cu(II) coordination sphere accompanied by a visible color change. Upon dissolution–recrystallization, the irreversible or reversible structural transformations among 2, 3, 4 and 6 could be driven by subtly controlling some factors such as solvent, pH, temperature and counter anion. The magnetic measurements for compounds 2–4 demonstrated that their magnetic behaviors are governed by antiferromagnetic interactions despite the fact that dissimilar exchange coupling pathways between neighboring Cu(II) ions are available in these three compounds.
中文翻译:

基于柔性两性离子苯并咪唑-二羧酸酯配体的Cu(II)配位化合物的可控组装:合成,结构多样性,可逆SCSC转变和磁性能
在不同条件下,将柔性两性离子配体1,3-双(2-羧乙基)苯并咪唑鎓(HL)与各种Cu(II)盐进行组装的系统研究得到了九种配位化合物:[Cu 2 L 2(NO 3)2 ]·CH 3 CN(1),[Cu 2 L 2(H 2 O)2 ](NO 3)2 ·5H 2 O(2),{[CuL(H 2 O)](NO 3)} n(3),[Cu 4 L 4(OH)2(H 2 O)2 ](NO 3)2 ·10H 2 O(4),[Cu 2 L 2(H 2 O)2 ](ClO 4)2(5),[CuL(H 2 O)3 ] 2 SO 4 ·7H 2 O(6),[Cu(HL)2 Cl 2 ](7),[Cu 2 L 2 Cl 2 ]·2H 2 O(8)和{[Cu 2 L 2 Cl 2 ]·2H 2 O} n(9)。化合物1和8为中性桨轮状双核簇,其中硝酸盐或氯化物为轴向配体,而化合物2和5由相同的[Cu 2 L 2(H 2 O)2 ] 2+阳离子单元组成,显示出带有轴向配位的水基配体但反荷阴离子不同的车轮基序。化合物3具有一维线性结构,其中两个相邻的Cu(II中心通过两个羧酸根基团和一个水族配体三重桥连。化合物4是由两个μ支撑的四核簇3 -bridged OH -基团和四个羧酸桥。化合物6和9是由二羧酸酯配体以两种不同配位方式构建的单核物质。化合物9假设一维环形链拓扑结构具有通过成对的灵活链接器传播的桨轮群集节点。仔细检查发现,柔性二羧酸酯配体的构象自由及其两个侧臂羧酸酯的不稳定结合模式对反应条件敏感,因此导致这些配位化合物的结构类型不同。之间的溶剂诱导可逆单晶到单晶(SCSC)变换1和3可以很容易地实现通过NO的穿梭运动3 -向外和向内的铜(II)协调球伴有可见的颜色变化。在溶解时再结晶,其中的不可逆的或可逆的结构转变2,3,4和6可以通过巧妙地控制一些因素,如溶剂,pH,温度和抗衡阴离子来驱动。化合物2–4的磁性测量结果表明,尽管这三种化合物中存在相邻Cu(II)离子之间存在互不相同的交换耦合途径,但它们的磁性仍受反铁磁相互作用的控制。
更新日期:2017-09-18
中文翻译:

基于柔性两性离子苯并咪唑-二羧酸酯配体的Cu(II)配位化合物的可控组装:合成,结构多样性,可逆SCSC转变和磁性能
在不同条件下,将柔性两性离子配体1,3-双(2-羧乙基)苯并咪唑鎓(HL)与各种Cu(II)盐进行组装的系统研究得到了九种配位化合物:[Cu 2 L 2(NO 3)2 ]·CH 3 CN(1),[Cu 2 L 2(H 2 O)2 ](NO 3)2 ·5H 2 O(2),{[CuL(H 2 O)](NO 3)} n(3),[Cu 4 L 4(OH)2(H 2 O)2 ](NO 3)2 ·10H 2 O(4),[Cu 2 L 2(H 2 O)2 ](ClO 4)2(5),[CuL(H 2 O)3 ] 2 SO 4 ·7H 2 O(6),[Cu(HL)2 Cl 2 ](7),[Cu 2 L 2 Cl 2 ]·2H 2 O(8)和{[Cu 2 L 2 Cl 2 ]·2H 2 O} n(9)。化合物1和8为中性桨轮状双核簇,其中硝酸盐或氯化物为轴向配体,而化合物2和5由相同的[Cu 2 L 2(H 2 O)2 ] 2+阳离子单元组成,显示出带有轴向配位的水基配体但反荷阴离子不同的车轮基序。化合物3具有一维线性结构,其中两个相邻的Cu(II中心通过两个羧酸根基团和一个水族配体三重桥连。化合物4是由两个μ支撑的四核簇3 -bridged OH -基团和四个羧酸桥。化合物6和9是由二羧酸酯配体以两种不同配位方式构建的单核物质。化合物9假设一维环形链拓扑结构具有通过成对的灵活链接器传播的桨轮群集节点。仔细检查发现,柔性二羧酸酯配体的构象自由及其两个侧臂羧酸酯的不稳定结合模式对反应条件敏感,因此导致这些配位化合物的结构类型不同。之间的溶剂诱导可逆单晶到单晶(SCSC)变换1和3可以很容易地实现通过NO的穿梭运动3 -向外和向内的铜(II)协调球伴有可见的颜色变化。在溶解时再结晶,其中的不可逆的或可逆的结构转变2,3,4和6可以通过巧妙地控制一些因素,如溶剂,pH,温度和抗衡阴离子来驱动。化合物2–4的磁性测量结果表明,尽管这三种化合物中存在相邻Cu(II)离子之间存在互不相同的交换耦合途径,但它们的磁性仍受反铁磁相互作用的控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号