当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of Building Blocks for Iterative Suzuki-Miyaura Reactions via Direct Bromination of Aryl Boronic Acids: One-Pot Total Syntheses of Dictyoterphenyls A and B
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-09-18 05:05:33 , DOI: 10.1002/adsc.201700733 Chun-Young Lee 1, 2 , Cheol-Hong Cheon 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-09-18 05:05:33 , DOI: 10.1002/adsc.201700733 Chun-Young Lee 1, 2 , Cheol-Hong Cheon 1
Affiliation
|
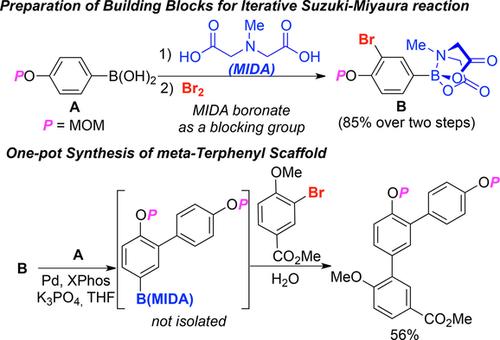
A highly efficient method for the preparation of 4-alkoxy-3-bromophenyl boronic acid N-methyliminodiacetic acid (MIDA) esters as building blocks in iterative Suzuki-Miyaura reactions from the 4-alkoxyphenylboronic acids is described using a boronic acid moiety as a blocking group in bromination reactions. With these MIDA boronates, the total syntheses of dictyoterphenyls A and B were developed in only two separate one-pot operations. Furthermore, we have developed a more practical protocol for the preparation of meta-terphenyl natural products by simply adding the second aryl halide and water to the reaction mixture via the controlled release technique.
中文翻译:

通过芳基硼酸的直接溴化反应制备铃木-Miyaura迭代反应的结构单元:一锅总的二氯三苯苯基A和B的合成
描述了一种高效的方法,该方法使用硼酸部分作为封闭剂,从4-烷氧基苯基硼酸制备Suzuki-Miyaura迭代Suzuki-Miyaura反应中作为结构单元的4-烷氧基-3-溴苯基硼酸N-甲基亚氨基二乙酸(MIDA)酯组中的溴化反应。使用这些MIDA硼酸盐,仅在两个单独的一锅操作中就开发了双邻苯二酚A和B的全部合成。此外,我们已经开发出了一种更实用的制备间三联苯天然产物的方案,只需通过控释技术将第二种芳基卤化物和水简单地添加到反应混合物中即可。
更新日期:2017-09-18
中文翻译:

通过芳基硼酸的直接溴化反应制备铃木-Miyaura迭代反应的结构单元:一锅总的二氯三苯苯基A和B的合成
描述了一种高效的方法,该方法使用硼酸部分作为封闭剂,从4-烷氧基苯基硼酸制备Suzuki-Miyaura迭代Suzuki-Miyaura反应中作为结构单元的4-烷氧基-3-溴苯基硼酸N-甲基亚氨基二乙酸(MIDA)酯组中的溴化反应。使用这些MIDA硼酸盐,仅在两个单独的一锅操作中就开发了双邻苯二酚A和B的全部合成。此外,我们已经开发出了一种更实用的制备间三联苯天然产物的方案,只需通过控释技术将第二种芳基卤化物和水简单地添加到反应混合物中即可。











































 京公网安备 11010802027423号
京公网安备 11010802027423号