当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structures of the N-Terminal Domain of PqsA in Complex with Anthraniloyl- and 6-Fluoroanthraniloyl-AMP: Substrate Activation in Pseudomonas Quinolone Signal (PQS) Biosynthesis
ChemBioChem ( IF 2.6 ) Pub Date : 2017-09-18 06:31:06 , DOI: 10.1002/cbic.201700374 Florian Witzgall 1 , Wiebke Ewert 1, 2 , Wulf Blankenfeldt 1, 3
ChemBioChem ( IF 2.6 ) Pub Date : 2017-09-18 06:31:06 , DOI: 10.1002/cbic.201700374 Florian Witzgall 1 , Wiebke Ewert 1, 2 , Wulf Blankenfeldt 1, 3
Affiliation
|
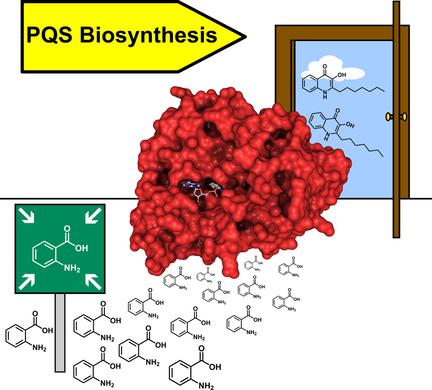
Gatekeeper of PQS biosynthesis unmasked: We determined the crystal structure of the N-terminal domain of PqsA, a member of the ANL superfamily that channels anthranilate into the biosynthetic pathway of the quorum-sensing (QS) molecule Pseudomonas quinolone signal (PQS). The structure might help in the development of specific QS inhibitors for therapeutic treatment.
中文翻译:

PqsA N末端域与蒽基和6-氟蒽基-AMP复合物中的结构:假单胞菌喹诺酮信号(PQS)生物合成中的底物激活。
PQS生物合成的守门员被揭露:我们确定了PqsA N端结构域的晶体结构,该结构是ANL超家族的成员,可将邻氨基苯甲酸引导至群体感应(QS)分子假单胞菌喹诺酮信号(PQS)的生物合成途径中。该结构可能有助于开发用于治疗的特定QS抑制剂。
更新日期:2017-09-18
中文翻译:

PqsA N末端域与蒽基和6-氟蒽基-AMP复合物中的结构:假单胞菌喹诺酮信号(PQS)生物合成中的底物激活。
PQS生物合成的守门员被揭露:我们确定了PqsA N端结构域的晶体结构,该结构是ANL超家族的成员,可将邻氨基苯甲酸引导至群体感应(QS)分子假单胞菌喹诺酮信号(PQS)的生物合成途径中。该结构可能有助于开发用于治疗的特定QS抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号