当前位置:
X-MOL 学术
›
JAMA Intern. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Malignant Neoplasms in Patients With Rheumatoid Arthritis Treated With Tumor Necrosis Factor Inhibitors, Tocilizumab, Abatacept, or Rituximab in Clinical Practice
JAMA Internal Medicine ( IF 39.0 ) Pub Date : 2017-11-01 , DOI: 10.1001/jamainternmed.2017.4332 Hjalmar Wadström 1 , Thomas Frisell 1 , Johan Askling 2 ,
JAMA Internal Medicine ( IF 39.0 ) Pub Date : 2017-11-01 , DOI: 10.1001/jamainternmed.2017.4332 Hjalmar Wadström 1 , Thomas Frisell 1 , Johan Askling 2 ,
Affiliation
|
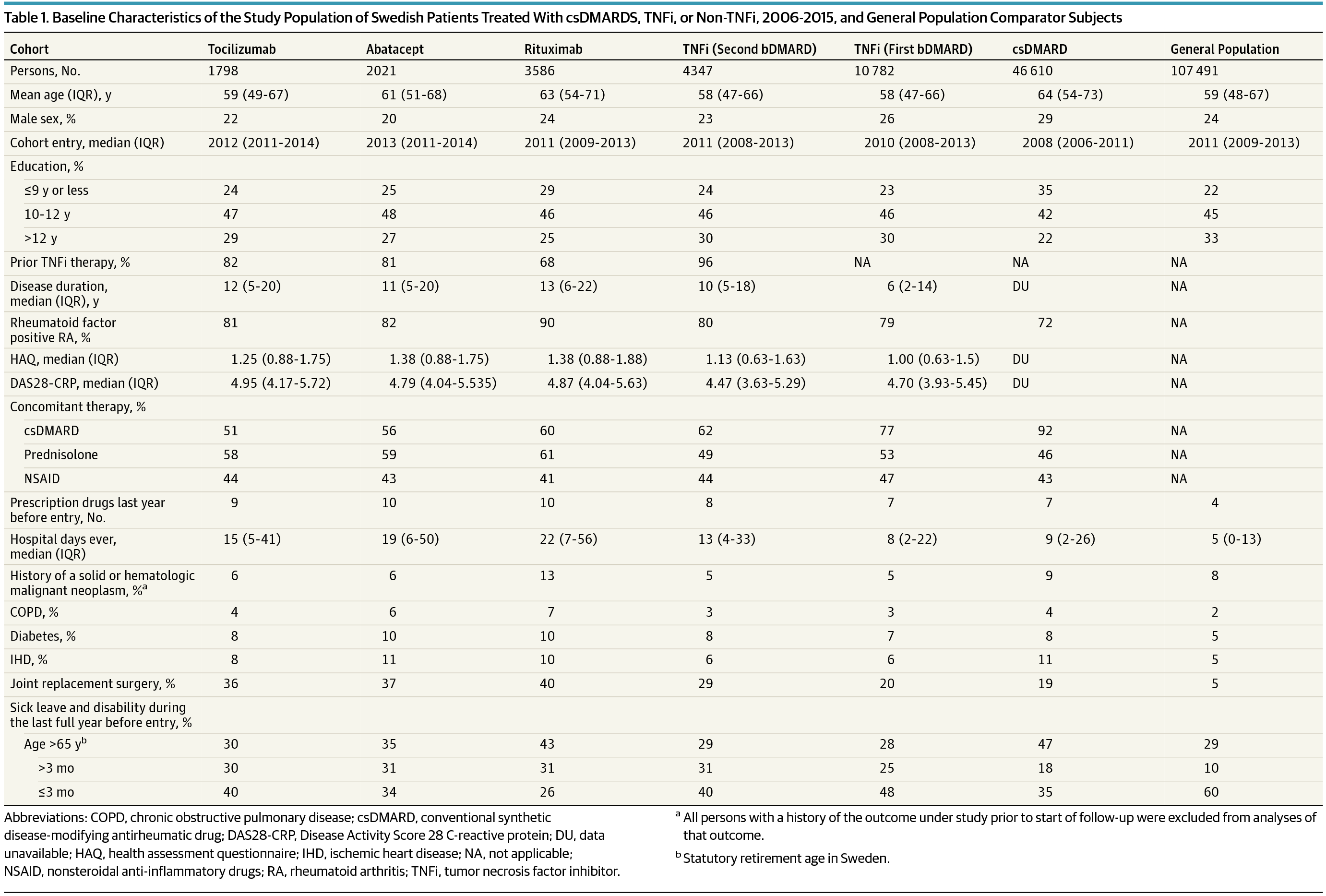
Importance Considering the widespread and increasing use of biological immunomodulators (biological disease-modifying antirheumatic drugs [bDMARDs]) to treat chronic inflammatory conditions, and the concern that immunomodulation may alter cancer risk and progression, the limited available data on use of these therapies as used in clinical practice and cancer risks are a concern. Objective To assess the risk of incident malignant neoplasms in patients with rheumatoid arthritis (RA) treated with bDMARDs. Design, Setting, and Participants This was a national register–based prospective cohort study of the public health care system in Sweden from 2006 to 2015. Cohorts of patients with RA initiating treatment with tocilizumab (n = 1798), abatacept (n = 2021), and rituximab (n = 3586), a tumor necrosis factor inhibitor (TNFi) as first-ever (n = 10 782) or second-ever (n = 4347) bDMARD, a biologics-naive cohort treated with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) (n = 46 610), and a general population comparator cohort (n = 107 491). Exposures Treatment with tocilizumab, abatacept, rituximab, or TNFi. Main Outcomes and Measures Outcomes included a first invasive solid or hematologic malignant neoplasm, or skin cancer. Hazard ratios were calculated using Cox-regression, adjusted for age, sex, disease and treatment characteristics, and educational level. Results We identified a total of 15 129 initiations of TNFi as the first or second bDMARD, 7405 initiations of other bDMARDs, and 46 610 csDMARD users. The mean age varied from 58 to 64 years, and the proportion of female patients varied from 71% to 80%, across the 7 cohorts under study. The observed numbers of events (crude incidence per 100 000 person-years) for a first invasive solid or hematologic malignant neoplasm were 50 (959) for tocilizumab, 61 (1026) for abatacept, 141 (1074) for rituximab, 478 (978) for initiators of TNFi as first bDMARD, and 169 (917) for TNFi as second bDMARD. There were no statistically significant differences between initiators of a first or second TNFi, or other bDMARDs, and bDMARD-naive RA for any of a total of 25 drug- and outcome-specific comparisons, with 1 exception (abatacept and increased risk of squamous cell skin cancer). Conclusions and Relevance The overall risk of cancer among patients with RA initiating TNFi as first or second bDMARD, tocilizumab, abatacept, or rituximab does not differ substantially from that of biologic drug–naive, csDMARD-treated patients with RA, although altered risks for specific cancer types, or those with longer latency, cannot be excluded.
中文翻译:

临床实践中使用肿瘤坏死因子抑制剂、托珠单抗、阿巴西普或利妥昔单抗治疗类风湿性关节炎患者的恶性肿瘤
重要性 考虑到生物免疫调节剂(生物疾病缓解性抗风湿药 [bDMARDs])在治疗慢性炎症方面的广泛使用和越来越多的使用,以及对免疫调节可能改变癌症风险和进展的担忧,关于使用这些疗法的可用数据有限在临床实践中,癌症风险是一个问题。目的 评估接受 bDMARDs 治疗的类风湿性关节炎 (RA) 患者发生恶性肿瘤的风险。设计、设置和参与者 这是 2006 年至 2015 年瑞典公共卫生保健系统的一项基于国家注册的前瞻性队列研究。 RA 患者队列开始使用托珠单抗(n = 1798)和阿巴西普(n = 2021)进行治疗, 和利妥昔单抗 (n = 3586), 肿瘤坏死因子抑制剂 (TNFi) 作为首次 (n = 10 782) 或第二次 (n = 4347) bDMARD,一个用常规合成疾病缓解抗风湿药 (csDMARDs) 治疗的未使用生物制剂的队列 (n = 46) 610)和一般人群比较队列(n = 107 491)。暴露 用托珠单抗、阿巴西普、利妥昔单抗或 TNFi 治疗。主要结果和措施 结果包括第一个侵袭性实体或血液系统恶性肿瘤,或皮肤癌。使用 Cox 回归计算风险比,并根据年龄、性别、疾病和治疗特征以及教育水平进行调整。结果 我们总共确定了 15129 次 TNFi 启动作为第一或第二 bDMARD,7405 次启动其他 bDMARD,以及 46610 名 csDMARD 使用者。平均年龄从 58 岁到 64 岁不等,在所研究的 7 个队列中,女性患者的比例从 71% 到 80% 不等。首次侵入性实体或血液系统恶性肿瘤的观察事件数(每 10 万人年的粗发病率)为托珠单抗 50 (959)、阿巴西普 61 (1026)、利妥昔单抗 141 (1074)、478 (978)对于作为第一个 bDMARD 的 TNFi 的引发剂,169 (917) 对于作为第二个 bDMARD 的 TNFi。对于总共 25 项药物和结果特异性比较中的任何一项,第一次或第二次 TNFi 或其他 bDMARDs 的起始者与 bDMARD 初治 RA 之间没有统计学显着差异,只有 1 个例外(阿巴西普和鳞状细胞癌风险增加)皮肤癌)。结论和相关性 RA 患者开始 TNFi 作为第一或第二 bDMARD、托珠单抗、阿巴西普、
更新日期:2017-11-01
中文翻译:

临床实践中使用肿瘤坏死因子抑制剂、托珠单抗、阿巴西普或利妥昔单抗治疗类风湿性关节炎患者的恶性肿瘤
重要性 考虑到生物免疫调节剂(生物疾病缓解性抗风湿药 [bDMARDs])在治疗慢性炎症方面的广泛使用和越来越多的使用,以及对免疫调节可能改变癌症风险和进展的担忧,关于使用这些疗法的可用数据有限在临床实践中,癌症风险是一个问题。目的 评估接受 bDMARDs 治疗的类风湿性关节炎 (RA) 患者发生恶性肿瘤的风险。设计、设置和参与者 这是 2006 年至 2015 年瑞典公共卫生保健系统的一项基于国家注册的前瞻性队列研究。 RA 患者队列开始使用托珠单抗(n = 1798)和阿巴西普(n = 2021)进行治疗, 和利妥昔单抗 (n = 3586), 肿瘤坏死因子抑制剂 (TNFi) 作为首次 (n = 10 782) 或第二次 (n = 4347) bDMARD,一个用常规合成疾病缓解抗风湿药 (csDMARDs) 治疗的未使用生物制剂的队列 (n = 46) 610)和一般人群比较队列(n = 107 491)。暴露 用托珠单抗、阿巴西普、利妥昔单抗或 TNFi 治疗。主要结果和措施 结果包括第一个侵袭性实体或血液系统恶性肿瘤,或皮肤癌。使用 Cox 回归计算风险比,并根据年龄、性别、疾病和治疗特征以及教育水平进行调整。结果 我们总共确定了 15129 次 TNFi 启动作为第一或第二 bDMARD,7405 次启动其他 bDMARD,以及 46610 名 csDMARD 使用者。平均年龄从 58 岁到 64 岁不等,在所研究的 7 个队列中,女性患者的比例从 71% 到 80% 不等。首次侵入性实体或血液系统恶性肿瘤的观察事件数(每 10 万人年的粗发病率)为托珠单抗 50 (959)、阿巴西普 61 (1026)、利妥昔单抗 141 (1074)、478 (978)对于作为第一个 bDMARD 的 TNFi 的引发剂,169 (917) 对于作为第二个 bDMARD 的 TNFi。对于总共 25 项药物和结果特异性比较中的任何一项,第一次或第二次 TNFi 或其他 bDMARDs 的起始者与 bDMARD 初治 RA 之间没有统计学显着差异,只有 1 个例外(阿巴西普和鳞状细胞癌风险增加)皮肤癌)。结论和相关性 RA 患者开始 TNFi 作为第一或第二 bDMARD、托珠单抗、阿巴西普、



























 京公网安备 11010802027423号
京公网安备 11010802027423号