当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Complexation and Redox Buffering of Iron(II) by Dissolved Organic Matter
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.est.7b03152 Ellen E. Daugherty 1 , Benjamin Gilbert 2 , Peter S. Nico 2 , Thomas Borch 1, 3
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.est.7b03152 Ellen E. Daugherty 1 , Benjamin Gilbert 2 , Peter S. Nico 2 , Thomas Borch 1, 3
Affiliation
|
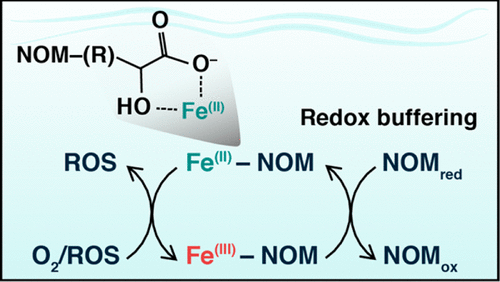
Iron (Fe) bioavailability depends upon its solubility and oxidation state, which are strongly influenced by complexation with natural organic matter (NOM). Despite observations of Fe(II)–NOM associations under conditions favorable for Fe oxidation, the molecular mechanisms by which NOM influences Fe(II) oxidation remain poorly understood. In this study, we used X-ray absorption spectroscopy to determine the coordination environment of Fe(II) associated with NOM (as-received and chemically reduced) at pH 7, and investigated the effect of NOM complexation on Fe(II) redox stability. Linear combination fitting of extended X-ray absorption fine structure (EXAFS) data using reference organic ligands demonstrated that Fe(II) was complexed primarily by carboxyl functional groups in reduced NOM. Functional groups more likely to preserve Fe(II) represent much smaller fractions of NOM-bound Fe(II). Fe(II) added to anoxic solutions of as-received NOM oxidized to Fe(III) and remained organically complexed. Iron oxidation experiments revealed that the presence of reduced NOM limited Fe(II) oxidation, with over 50% of initial Fe(II) remaining after 4 h. These results suggest reduced NOM may preserve Fe(II) by functioning both as redox buffer and complexant, which may help explain the presence of Fe(II) in oxic circumneutral waters.
中文翻译:

溶解性有机物对铁(II)的络合和氧化还原缓冲作用
铁(Fe)的生物利用度取决于它的溶解度和氧化态,这与天然有机物(NOM)的络合强烈影响。尽管在有利于铁氧化的条件下观察到Fe(II)-NOM缔合,但对NOM影响Fe(II)氧化的分子机制仍然知之甚少。在这项研究中,我们使用X射线吸收光谱法确定了pH值为7时与NOM(按原样还原和化学还原)相关的Fe(II)的配位环境,并研究了NOM络合对Fe(II)氧化还原稳定性的影响。使用参考有机配体的扩展X射线吸收精细结构(EXAFS)数据的线性组合拟合表明,Fe(II)主要被还原的NOM中的羧基官能团络合。更有可能保留Fe(II)的官能团代表与NOM结合的Fe(II)的比例要小得多。将Fe(II)添加到按原样的NOM氧化为Fe(III)的缺氧溶液中,并保持有机络合状态。铁的氧化实验表明,还原型NOM的存在限制了Fe(II)的氧化,在4 h后剩余了超过50%的初始Fe(II)。这些结果表明,减少的NOM可以通过充当氧化还原缓冲剂和络合物来保留Fe(II),这可能有助于解释在含氧的中性水域中Fe(II)的存在。
更新日期:2017-09-18
中文翻译:

溶解性有机物对铁(II)的络合和氧化还原缓冲作用
铁(Fe)的生物利用度取决于它的溶解度和氧化态,这与天然有机物(NOM)的络合强烈影响。尽管在有利于铁氧化的条件下观察到Fe(II)-NOM缔合,但对NOM影响Fe(II)氧化的分子机制仍然知之甚少。在这项研究中,我们使用X射线吸收光谱法确定了pH值为7时与NOM(按原样还原和化学还原)相关的Fe(II)的配位环境,并研究了NOM络合对Fe(II)氧化还原稳定性的影响。使用参考有机配体的扩展X射线吸收精细结构(EXAFS)数据的线性组合拟合表明,Fe(II)主要被还原的NOM中的羧基官能团络合。更有可能保留Fe(II)的官能团代表与NOM结合的Fe(II)的比例要小得多。将Fe(II)添加到按原样的NOM氧化为Fe(III)的缺氧溶液中,并保持有机络合状态。铁的氧化实验表明,还原型NOM的存在限制了Fe(II)的氧化,在4 h后剩余了超过50%的初始Fe(II)。这些结果表明,减少的NOM可以通过充当氧化还原缓冲剂和络合物来保留Fe(II),这可能有助于解释在含氧的中性水域中Fe(II)的存在。











































 京公网安备 11010802027423号
京公网安备 11010802027423号