Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Confinement of iodides in carbon porosity to prevent from positive electrode oxidation in high voltage aqueous hybrid electrochemical capacitors
Carbon ( IF 10.5 ) Pub Date : 2017-12-01 , DOI: 10.1016/j.carbon.2017.09.060 Patryk Przygocki , Qamar Abbas , Paulina Babuchowska , François Béguin
Carbon ( IF 10.5 ) Pub Date : 2017-12-01 , DOI: 10.1016/j.carbon.2017.09.060 Patryk Przygocki , Qamar Abbas , Paulina Babuchowska , François Béguin
|
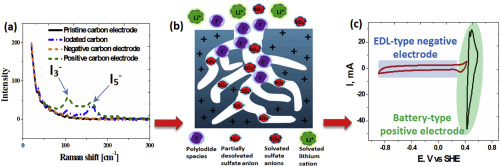
Abstract Hybridization of battery-type positive and capacitor-type negative activated carbon (AC) electrodes has been realized in a carbon/carbon electrochemical capacitor by adding potassium iodide (0.5 mol L−1 KI) to aqueous manganese sulfate (2 mol L−1 MnSO4) electrolyte. Raman spectroscopy proved that polyiodides are formed inside the porosity of the positive carbon electrode under polarization in MnSO4+KI electrolyte, and give rise to an interface being at the origin of the battery-like behavior of this electrode. By contrast, the capacitive negative carbon electrode displays a wider potential range, which allows the cell to operate up to 1.5 V. When the hybrid cell is charged at 0.1 A g−1 up to 1.5 V, it exhibits a higher capacitance of 81 F g−1 (expressed per total mass of active carbon material) compared to 41 F g−1 for the symmetric cell in MnSO4. During potentiostatic floating at 1.5 V, the hybrid cell in MnSO4+KI demonstrates remarkably stable capacitance and resistance. Temperature-programmed desorption (TPD) analyses and nitrogen adsorption on the positive electrode of this cell after floating reveal noticeably reduced oxidation as compared to the positive electrode of the cell floated in MnSO4. Such reduced oxidation is owing to the redox couples associated with polyiodides confined in the porosity of the electrode which contribute to stabilize its potential well below the water oxidation limit.
中文翻译:

将碘化物限制在碳孔隙中以防止高压水性混合电化学电容器中的正极氧化
摘要 通过将碘化钾 (0.5 mol L−1 KI) 添加到硫酸锰水溶液 (2 mol L−1 MnSO4) 电解质。拉曼光谱证明,多碘化物在 MnSO4+KI 电解液中极化下在正极碳电极的孔隙内形成,并在该电极的类似电池行为的起源处产生界面。相比之下,电容负碳电极显示出更宽的电位范围,允许电池在高达 1.5 V 的电压下工作。当混合电池以 0.1 A g-1 充电至 1.5 V 时,与 MnSO4 中对称电池的 41 F g-1 相比,它表现出更高的电容 81 F g-1(按活性炭材料的总质量表示)。在 1.5 V 的恒电位浮动期间,MnSO4+KI 中的混合电池表现出非常稳定的电容和电阻。程序升温解吸 (TPD) 分析和漂浮后该电池正极上的氮吸附显示,与漂浮在 MnSO4 中的电池正极相比,氧化显着减少。这种减少的氧化是由于与限制在电极孔隙率中的多碘化物相关的氧化还原对,这有助于将其电位稳定在远低于水氧化极限的水平。程序升温解吸 (TPD) 分析和漂浮后该电池正极上的氮吸附显示,与漂浮在 MnSO4 中的电池正极相比,氧化显着减少。这种减少的氧化是由于与限制在电极孔隙率中的多碘化物相关的氧化还原对,这有助于将其电位稳定在远低于水氧化极限的水平。程序升温解吸 (TPD) 分析和漂浮后该电池正极上的氮吸附显示,与漂浮在 MnSO4 中的电池正极相比,氧化显着减少。这种减少的氧化是由于与限制在电极孔隙率中的多碘化物相关的氧化还原对,这有助于将其电位稳定在远低于水氧化极限的水平。
更新日期:2017-12-01
中文翻译:

将碘化物限制在碳孔隙中以防止高压水性混合电化学电容器中的正极氧化
摘要 通过将碘化钾 (0.5 mol L−1 KI) 添加到硫酸锰水溶液 (2 mol L−1 MnSO4) 电解质。拉曼光谱证明,多碘化物在 MnSO4+KI 电解液中极化下在正极碳电极的孔隙内形成,并在该电极的类似电池行为的起源处产生界面。相比之下,电容负碳电极显示出更宽的电位范围,允许电池在高达 1.5 V 的电压下工作。当混合电池以 0.1 A g-1 充电至 1.5 V 时,与 MnSO4 中对称电池的 41 F g-1 相比,它表现出更高的电容 81 F g-1(按活性炭材料的总质量表示)。在 1.5 V 的恒电位浮动期间,MnSO4+KI 中的混合电池表现出非常稳定的电容和电阻。程序升温解吸 (TPD) 分析和漂浮后该电池正极上的氮吸附显示,与漂浮在 MnSO4 中的电池正极相比,氧化显着减少。这种减少的氧化是由于与限制在电极孔隙率中的多碘化物相关的氧化还原对,这有助于将其电位稳定在远低于水氧化极限的水平。程序升温解吸 (TPD) 分析和漂浮后该电池正极上的氮吸附显示,与漂浮在 MnSO4 中的电池正极相比,氧化显着减少。这种减少的氧化是由于与限制在电极孔隙率中的多碘化物相关的氧化还原对,这有助于将其电位稳定在远低于水氧化极限的水平。程序升温解吸 (TPD) 分析和漂浮后该电池正极上的氮吸附显示,与漂浮在 MnSO4 中的电池正极相比,氧化显着减少。这种减少的氧化是由于与限制在电极孔隙率中的多碘化物相关的氧化还原对,这有助于将其电位稳定在远低于水氧化极限的水平。











































 京公网安备 11010802027423号
京公网安备 11010802027423号