当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Silane–Acrylate Chemistry for Regulating Network Formation in Radical Photopolymerization
Macromolecules ( IF 5.5 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.macromol.7b01399 Johannes Steindl 1, 2 , Thomas Koch 3 , Norbert Moszner 2, 4 , Christian Gorsche 1, 2
Macromolecules ( IF 5.5 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.macromol.7b01399 Johannes Steindl 1, 2 , Thomas Koch 3 , Norbert Moszner 2, 4 , Christian Gorsche 1, 2
Affiliation
|
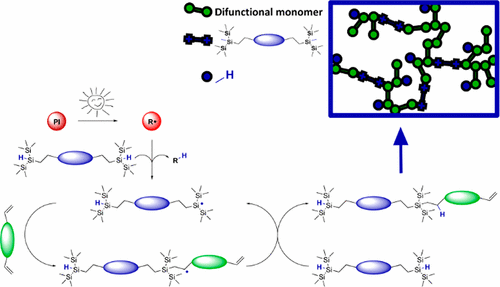
Photoinitiated silane–ene chemistry has the potential to pave the way toward spatially resolved organosilicon compounds, which might find application in biomedicine, microelectronics, and other advanced fields. Moreover, this approach could serve as a viable alternative to the popular photoinitiated thiol–ene chemistry, which gives access to defined and functional photopolymer networks. A difunctional bis(trimethylsilyl)silane with abstractable hydrogens (DSiH) was successfully synthesized in a simple one-pot procedure. The radical reactivity of DSiH with various homopolymerizable monomers (i.e., (meth)acrylate, vinyl ester, acrylamide) was assessed via 1H NMR spectroscopic studies. DSiH shows good reactivity with acrylates and vinyl esters. The most promising silane–acrylate system was further investigated in cross-linking formulations toward its reactivity (e.g., heat of polymerization, curing time, occurrence of gelation, double-bond conversion) and compared to state-of-the-art thiol–acrylate resins. The storage stability of prepared resin formulations is greatly improved for silane–acrylate systems vs thiol–ene resins. Double-bond conversion at the gel point (DBCgel) and overall DBC were increased, and polymerization-induced shrinkage stress has been significantly reduced with the introduction of silane–acrylate chemistry. Resulting photopolymer networks exhibit a homogeneous network architecture (indicated by a narrow glass transition) that can be tuned by varying silane concentration, and this confirms the postulated regulation of radical network formation. Similar to thiol–acrylate networks, this leads to more flexible photopolymer networks with increased elongation at break and improved impact resistance. Additionally, swelling tests indicate a high gel fraction for silane–acrylate photopolymers.
中文翻译:

硅烷-丙烯酸酯化学用于调节自由基光聚合中的网络形成
光引发的硅烷化学有可能为空间分解的有机硅化合物铺平道路,有机硅化合物可能会在生物医学,微电子学和其他先进领域中得到应用。而且,这种方法可以替代流行的光引发的硫醇-烯化学方法,该方法可以使用已定义的功能性光敏聚合物网络。通过简单的一锅法成功合成了具有可提取氢的双官能双(三甲基甲硅烷基)硅烷(DSiH)。DSiH与各种可均聚单体(即(甲基)丙烯酸酯,乙烯基酯,丙烯酰胺)的自由基反应性通过1进行评估1 H NMR光谱学研究。DSiH显示出与丙烯酸酯和乙烯基酯的良好反应性。在交联配方中进一步研究了最有希望的硅烷-丙烯酸酯体系是否具有反应活性(例如,聚合热,固化时间,发生胶凝,双键转化),并与最先进的硫醇-丙烯酸酯进行了比较。树脂。与硫醇烯树脂相比,硅烷-丙烯酸酯体系制备的树脂配方的储存稳定性大大提高。凝胶点的双键转化(DBC凝胶)和整体DBC的增加,并且由于引入了硅烷-丙烯酸酯化学反应,聚合引发的收缩应力已大大降低。所得的光敏聚合物网络显示出均一的网络结构(由狭窄的玻璃化转变指示),可以通过改变硅烷浓度进行调整,这证实了自由基网络形成的假设调控。与硫醇丙烯酸酯网络相似,这会导致更灵活的光聚合物网络,其断裂伸长率增加,抗冲击性提高。另外,溶胀测试表明硅烷-丙烯酸酯光敏聚合物的凝胶分数很高。
更新日期:2017-09-18
中文翻译:

硅烷-丙烯酸酯化学用于调节自由基光聚合中的网络形成
光引发的硅烷化学有可能为空间分解的有机硅化合物铺平道路,有机硅化合物可能会在生物医学,微电子学和其他先进领域中得到应用。而且,这种方法可以替代流行的光引发的硫醇-烯化学方法,该方法可以使用已定义的功能性光敏聚合物网络。通过简单的一锅法成功合成了具有可提取氢的双官能双(三甲基甲硅烷基)硅烷(DSiH)。DSiH与各种可均聚单体(即(甲基)丙烯酸酯,乙烯基酯,丙烯酰胺)的自由基反应性通过1进行评估1 H NMR光谱学研究。DSiH显示出与丙烯酸酯和乙烯基酯的良好反应性。在交联配方中进一步研究了最有希望的硅烷-丙烯酸酯体系是否具有反应活性(例如,聚合热,固化时间,发生胶凝,双键转化),并与最先进的硫醇-丙烯酸酯进行了比较。树脂。与硫醇烯树脂相比,硅烷-丙烯酸酯体系制备的树脂配方的储存稳定性大大提高。凝胶点的双键转化(DBC凝胶)和整体DBC的增加,并且由于引入了硅烷-丙烯酸酯化学反应,聚合引发的收缩应力已大大降低。所得的光敏聚合物网络显示出均一的网络结构(由狭窄的玻璃化转变指示),可以通过改变硅烷浓度进行调整,这证实了自由基网络形成的假设调控。与硫醇丙烯酸酯网络相似,这会导致更灵活的光聚合物网络,其断裂伸长率增加,抗冲击性提高。另外,溶胀测试表明硅烷-丙烯酸酯光敏聚合物的凝胶分数很高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号