当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold-Catalyzed Synthesis of 1-(Indol-3-yl)carbazoles: Selective 1,2-Alkyl vs 1,2-Vinyl Migration
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.orglett.7b02303 Anisley Suárez 1 , Samuel Suárez-Pantiga 1 , Olalla Nieto-Faza 2 , Roberto Sanz 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.orglett.7b02303 Anisley Suárez 1 , Samuel Suárez-Pantiga 1 , Olalla Nieto-Faza 2 , Roberto Sanz 1
Affiliation
|
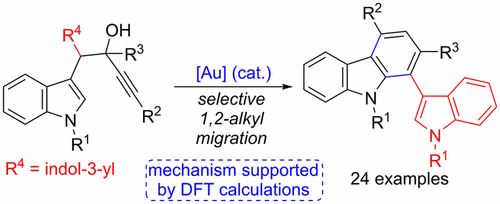
Gold(III)-catalyzed cycloisomerization of α-bis(indol-3-yl)methyl alkynols selectively affords 1-(indol-3-yl)carbazoles, in a transformation that takes place through a selective 1,2-alkyl vs 1,2-vinyl migration step in the vinyl-gold intermediate generated from the initial 5-endo-spirocyclization. The reaction proceeds well with either tertiary or secondary starting alkynols as well as with a wide variety of alkyne substituents. The key role of the other indol-3-yl substituent for the unexpected selectivity in the 1,2 rearrangement has also been supported by DFT calculations that reveal a low barrier, two-step mechanism in the alkyl migration path where the second indole significantly stabilizes a carbocationic intermediate.
中文翻译:

金催化的1-(吲哚-3-基)咔唑的合成:选择性1,2-烷基与1,2-乙烯基迁移
金(III)催化的α-双(吲哚-3-基)甲基炔醇的环异构化选择性地产生了1-(吲哚-3-基)咔唑,其转化是通过选择性的1,2-烷基与1进行的,从最初的5-内-螺环化反应生成的乙烯基-金中间体中的2-乙烯基迁移步骤。该反应与叔或仲起始炔醇以及多种炔烃取代基一起进行得很好。DFT计算还支持了另一个吲哚-3-基取代基对于1,2重排中的意外选择性的关键作用,该计算揭示了烷基迁移路径中的低阻隔两步机理,其中第二个吲哚显着稳定碳阳离子中间体。
更新日期:2017-09-18
中文翻译:

金催化的1-(吲哚-3-基)咔唑的合成:选择性1,2-烷基与1,2-乙烯基迁移
金(III)催化的α-双(吲哚-3-基)甲基炔醇的环异构化选择性地产生了1-(吲哚-3-基)咔唑,其转化是通过选择性的1,2-烷基与1进行的,从最初的5-内-螺环化反应生成的乙烯基-金中间体中的2-乙烯基迁移步骤。该反应与叔或仲起始炔醇以及多种炔烃取代基一起进行得很好。DFT计算还支持了另一个吲哚-3-基取代基对于1,2重排中的意外选择性的关键作用,该计算揭示了烷基迁移路径中的低阻隔两步机理,其中第二个吲哚显着稳定碳阳离子中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号