当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio- and Stereospecific Construction of 3a-(1H-Indol-3-yl)pyrrolidinoindolines and Application to the Formal Syntheses of Gliocladins B and C
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.orglett.7b02425 Honghui Lei 1 , Lushun Wang 1 , Zhengshuang Xu 1 , Tao Ye 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.orglett.7b02425 Honghui Lei 1 , Lushun Wang 1 , Zhengshuang Xu 1 , Tao Ye 1
Affiliation
|
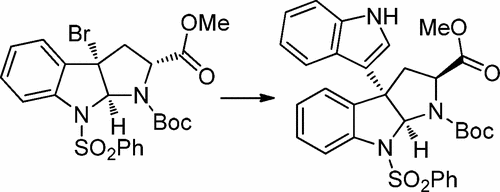
A one-pot regio- and stereospecific strategy for the construction of 3a-(3-indolyl)-hexahydropyrrolo[2,3-b]indoles based on the condensation of an indole and an in situ generated cyclopropylazetoindoline has been developed. This unified strategy works with a variety of substituted indoles to produce 3a-(3-indolyl)hexahydropyrrolo[2,3-b]indole products in high yields. The utility of this transformation was highlighted in the formal total syntheses of gliocladins B and C.
中文翻译:

3a-(1 H-吲哚-3-基)吡咯烷二氢吲哚的区域和立体特异性构建及其在胶质环丁素B和C的形式合成中的应用
已经开发了一种基于吲哚和原位生成的环丙基氮杂吲哚并吲哚的缩合反应构建3a-(3-吲哚基)-六氢吡咯并[2,3- b ]吲哚的一锅区域和立体定向策略。这种统一的策略可与多种取代的吲哚配合使用,以高收率生产3a-(3-吲哚基)六氢吡咯并[2,3- b ]吲哚产物。gliocladins B和C的正式总合成中突显了这种转化的效用。
更新日期:2017-09-18
中文翻译:

3a-(1 H-吲哚-3-基)吡咯烷二氢吲哚的区域和立体特异性构建及其在胶质环丁素B和C的形式合成中的应用
已经开发了一种基于吲哚和原位生成的环丙基氮杂吲哚并吲哚的缩合反应构建3a-(3-吲哚基)-六氢吡咯并[2,3- b ]吲哚的一锅区域和立体定向策略。这种统一的策略可与多种取代的吲哚配合使用,以高收率生产3a-(3-吲哚基)六氢吡咯并[2,3- b ]吲哚产物。gliocladins B和C的正式总合成中突显了这种转化的效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号